Mechanisms and clinical landscape of N6-methyladenosine (m6A) RNA modification in gastrointestinal tract cancers
- PMID: 38856795
- PMCID: PMC11254988
- DOI: 10.1007/s11010-024-05040-x
Mechanisms and clinical landscape of N6-methyladenosine (m6A) RNA modification in gastrointestinal tract cancers
Abstract
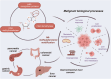
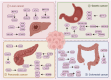
Epigenetics encompasses reversible and heritable chemical modifications of non-nuclear DNA sequences, including DNA and RNA methylation, histone modifications, non-coding RNA modifications, and chromatin rearrangements. In addition to well-studied DNA and histone methylation, RNA methylation has emerged as a hot topic in biological sciences over the past decade. N6-methyladenosine (m6A) is the most common and abundant modification in eukaryotic mRNA, affecting all RNA stages, including transcription, translation, and degradation. Advances in high-throughput sequencing technologies made it feasible to identify the chemical basis and biological functions of m6A RNA. Dysregulation of m6A levels and associated modifying proteins can both inhibit and promote cancer, highlighting the importance of the tumor microenvironment in diverse biological processes. Gastrointestinal tract cancers, including gastric, colorectal, and pancreatic cancers, are among the most common and deadly malignancies in humans. Growing evidence suggests a close association between m6A levels and the progression of gastrointestinal tumors. Global m6A modification levels are substantially modified in gastrointestinal tumor tissues and cell lines compared to healthy tissues and cells, possibly influencing various biological behaviors such as tumor cell proliferation, invasion, metastasis, and drug resistance. Exploring the diagnostic and therapeutic potential of m6A-related proteins is critical from a clinical standpoint. Developing more specific and effective m6A modulators offers new options for treating these tumors and deeper insights into gastrointestinal tract cancers.
Keywords: Clinical applications; Expression level; Gastrointestinal tract cancers; Mechanism; m6A.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
N6-methyladenosine (m6A) modification in gynecological malignancies.J Cell Physiol. 2022 Sep;237(9):3465-3479. doi: 10.1002/jcp.30828. Epub 2022 Jul 8. J Cell Physiol. 2022. PMID: 35802474 Review.
-
The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis.Mol Cancer. 2020 Feb 28;19(1):44. doi: 10.1186/s12943-020-01172-y. Mol Cancer. 2020. PMID: 32111216 Free PMC article. Review.
-
The Mechanism and Latest Progress of m6A Methylation in the Progression of Pancreatic Cancer.Int J Biol Sci. 2025 Jan 13;21(3):1187-1201. doi: 10.7150/ijbs.104407. eCollection 2025. Int J Biol Sci. 2025. PMID: 39897038 Free PMC article. Review.
-
N6-methyladenosine functions and its role in skin cancer.Exp Dermatol. 2023 Jan;32(1):4-12. doi: 10.1111/exd.14696. Epub 2022 Nov 13. Exp Dermatol. 2023. PMID: 36314059 Review.
-
Mechanism of RNA modification N6-methyladenosine in human cancer.Mol Cancer. 2020 Jun 8;19(1):104. doi: 10.1186/s12943-020-01216-3. Mol Cancer. 2020. PMID: 32513173 Free PMC article. Review.
Cited by
-
Research progress on m6A and drug resistance in gastrointestinal tumors.Front Pharmacol. 2025 Apr 28;16:1565738. doi: 10.3389/fphar.2025.1565738. eCollection 2025. Front Pharmacol. 2025. PMID: 40356985 Free PMC article. Review.
-
Epigenetically Regulating Non-coding RNAs in Colorectal Cancer: Promises and Potentials.Middle East J Dig Dis. 2025 Jan;17(1):40-53. doi: 10.34172/mejdd.2025.404. Epub 2025 Jan 31. Middle East J Dig Dis. 2025. PMID: 40322568 Free PMC article. Review.
-
Epitranscriptomic RNA m6A Modification in Cancer Therapy Resistance: Challenges and Unrealized Opportunities.Adv Sci (Weinh). 2024 Dec 11;12(4):e2403936. doi: 10.1002/advs.202403936. Online ahead of print. Adv Sci (Weinh). 2024. PMID: 39661414 Free PMC article. Review.
-
Epigenetic modifications in cardiac fibrosis: recent evidence of new pharmacological targets.Front Mol Biosci. 2025 May 2;12:1583446. doi: 10.3389/fmolb.2025.1583446. eCollection 2025. Front Mol Biosci. 2025. PMID: 40384946 Free PMC article. Review.
-
Implications of miRNA-590 dysregulation in digestive tract malignancies: mechanistic aspects and clinical significance.Mol Biol Rep. 2025 Jun 5;52(1):551. doi: 10.1007/s11033-025-10656-3. Mol Biol Rep. 2025. PMID: 40471218 Review.
References
-
- Stukalin I, Ahmed NS, Fundytus AM, Qian AS, Coward S, Kaplan GG, Hilsden RJ, Burak KW, Lee JK, Singh S, Ma C Trends and projections in national united states health care spending for gastrointestinal malignancies (1996–2030) Gastroenterology. 2022;162:1098–1110.e2. doi: 10.1053/j.gastro.2021.12.244. - DOI - PMC - PubMed
-
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

