Round and flat zygomatic implants: effectiveness after a 3‑year follow‑up non‑interventional study
- PMID: 38856876
- PMCID: PMC11164844
- DOI: 10.1186/s40729-024-00548-9
Round and flat zygomatic implants: effectiveness after a 3‑year follow‑up non‑interventional study
Abstract
Purpose: This non-interventional study investigates variations in the type and frequency of late complications linked to novel zygomatic implant designs, installed adhering to the Zygoma Anatomy-Guided Approach (ZAGA) concept, over an extended follow-up period of at least 3 years.
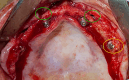
Methods: Consecutive patients presenting indications for treatment with ZIs were treated according to ZAGA recommendations. Implants were immediately loaded. The ORIS success criteria for prosthetic offset, stability, sinus changes and soft-tissue status were used to evaluate the outcome.
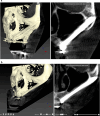
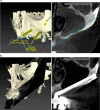
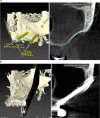
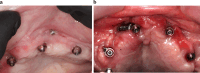
Results: Twenty patients were treated. Ten patients received two ZIs and regular implants; one received three ZIs plus regular implants, and nine received four ZIs. Fifty-nine ZIs were placed: thirty-six (61%) Straumann ZAGA-Flat implants and twenty-three (39%) Straumann ZAGA-Round implants. Four patients (20%) presented earlier sinus floor discontinuities. Fifteen patients (75%) had prior sinus opacities. Nineteen patients were followed for between 38 and 53 months (mean 46.5 months). One patient dropped out after 20 months. When comparing pre-surgical CBCT with post-surgical CBCT, 84.7% of the sites presented identical or less sinus opacity; nine locations (15%) showed decreased, and another nine increased (15%) post-surgical sinus opacity. Fifty-three ZIs (89.8%) maintained stable soft tissue. Six ZIs had recessions with no signs of infection. ZIs and prosthesis survival rate was 100%.
Conclusions: The study highlights the effectiveness of ZAGA-based zygomatic implant rehabilitations using Round and Flat designs. Despite patient number constraints, minimal changes in the frequency of late complications from the 1-year follow-up were observed. 100% implant and prosthesis survival rate over a mean follow-up of 46.5 months is reported.
Keywords: ORIS criteria; ZAGA; ZAGA implants; ZAGA-flat; ZAGA-round; Zygomatic implant late complications; Zygomatic implants.
© 2024. The Author(s).
Conflict of interest statement
Carlos Aparicio is the founding president of Zygoma ZAGA Centers and occasionally provides CE activities for NobelBiocare, Straumann Group, SouthernImplants and Versah Osseodensification Company. Waldemar Polido occasionally performs CE activities for Straumann Group and Geistlich Biomaterials. Bilal Al-Nawas occasionally performs CE activities and obtains research grants for StraumannGroup, BEGO, Camlog, Dentsply and Geistlich Biomaterials. All other authors declare that they have no conflicts of interest.
Figures









Similar articles
-
Round and flat zygomatic implants: effectiveness after a 1-year follow-up non-interventional study.Int J Implant Dent. 2022 Apr 1;8(1):13. doi: 10.1186/s40729-022-00412-8. Int J Implant Dent. 2022. PMID: 35359196 Free PMC article.
-
Comparative Clinical Outcomes of Two Different Zygomatic Implant Protocols for the Rehabilitation of Severely Atrophic Maxillae: A Retrospective Analysis.Int J Periodontics Restorative Dent. 2025 Jan 7;0(0):1-23. doi: 10.11607/prd.7493. Online ahead of print. Int J Periodontics Restorative Dent. 2025. PMID: 39775712
-
Zygomatic implants placed using the zygomatic anatomy-guided approach versus the classical technique: a proposed system to report rhinosinusitis diagnosis.Clin Implant Dent Relat Res. 2014 Oct;16(5):627-42. doi: 10.1111/cid.12047. Epub 2013 Mar 6. Clin Implant Dent Relat Res. 2014. PMID: 23464749
-
ORIS Criteria of Success for the Zygoma-Related Rehabilitation: The (Revisited) Zygoma Success Code.Int J Oral Maxillofac Implants. 2020 Mar/Apr;35(2):366-378. doi: 10.11607/jomi.7488. Int J Oral Maxillofac Implants. 2020. PMID: 32142574
-
ZAGA Protocol for Quadruple Zygoma Implant Installation.Atlas Oral Maxillofac Surg Clin North Am. 2025 Sep;33(2):149-157. doi: 10.1016/j.cxom.2025.04.004. Epub 2025 Jul 5. Atlas Oral Maxillofac Surg Clin North Am. 2025. PMID: 40818863 Review. No abstract available.
Cited by
-
Workflow for Maxilla/Mandible Individual [Mai®] Implant by Integra Implants-How Individual Implants Are Manufactured.Biomedicines. 2024 Aug 6;12(8):1773. doi: 10.3390/biomedicines12081773. Biomedicines. 2024. PMID: 39200237 Free PMC article.
References
-
- Aparicio C, Branemark PI, Keller EE, Olivé J. Reconstruction of the premaxilla with autogenous lliac bone in combination with osseointegrated implants. Int J Oral Maxillofac Implants. 1993;8:1–15.
-
- Jensen OT, Brownd C, Blacker J. Nasofacial prostheses supported by osseointegrated implants. Int J Oral Maxillofac Implants. 1992;7(2):203–211. - PubMed
-
- Sáez-Alcaide LM, Cortés-Bretón-Brinkmann J, Sánchez-Labrador L, Pérez-González F, Forteza-López A, Molinero-Mourelle P, et al. Patient-reported outcomes in patients with severe maxillary bone atrophy restored with zygomatic implant-supported complete dental prostheses: a systematic review. Acta Odontol Scand. 2022;80(5):363–373. doi: 10.1080/00016357.2021.2018494. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

