Pyruvate kinase M2 sustains cardiac mitochondrial quality surveillance in septic cardiomyopathy by regulating prohibitin 2 abundance via S91 phosphorylation
- PMID: 38856931
- PMCID: PMC11335292
- DOI: 10.1007/s00018-024-05253-9
Pyruvate kinase M2 sustains cardiac mitochondrial quality surveillance in septic cardiomyopathy by regulating prohibitin 2 abundance via S91 phosphorylation
Abstract
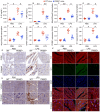
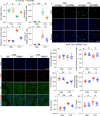
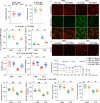
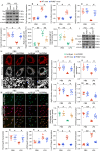
The endogenous mitochondrial quality control (MQC) system serves to protect mitochondria against cellular stressors. Although mitochondrial dysfunction contributes to cardiac damage during many pathological conditions, the regulatory signals influencing MQC disruption during septic cardiomyopathy (SC) remain unclear. This study aimed to investigate the involvement of pyruvate kinase M2 (PKM2) and prohibitin 2 (PHB2) interaction followed by MQC impairment in the pathogenesis of SC. We utilized LPS-induced SC models in PKM2 transgenic (PKM2TG) mice, PHB2S91D-knockin mice, and PKM2-overexpressing HL-1 cardiomyocytes. After LPS-induced SC, cardiac PKM2 expression was significantly downregulated in wild-type mice, whereas PKM2 overexpression in vivo sustained heart function, suppressed myocardial inflammation, and attenuated cardiomyocyte death. PKM2 overexpression relieved sepsis-related mitochondrial damage via MQC normalization, evidenced by balanced mitochondrial fission/fusion, activated mitophagy, restored mitochondrial biogenesis, and inhibited mitochondrial unfolded protein response. Docking simulations, co-IP, and domain deletion mutant protein transfection experiments showed that PKM2 phosphorylates PHB2 at Ser91, preventing LPS-mediated PHB2 degradation. Additionally, the A domain of PKM2 and the PHB domain of PHB2 are required for PKM2-PHB2 binding and PHB2 phosphorylation. After LPS exposure, expression of a phosphorylation-defective PHB2S91A mutant negated the protective effects of PKM2 overexpression. Moreover, knockin mice expressing a phosphorylation-mimetic PHB2S91D mutant showed improved heart function, reduced inflammation, and preserved mitochondrial function following sepsis induction. Abundant PKM2 expression is a prerequisite to sustain PKM2-PHB2 interaction which is a key element for preservation of PHB2 phosphorylation and MQC, presenting novel interventive targets for the treatment of septic cardiomyopathy.
Keywords: MQC; Mitochondria; PHB2; PKM2; Septic cardiomyopathy.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no conflict of interest.
Figures





Similar articles
-
Pyruvate kinase M2 sustains cardiac mitochondrial integrity in septic cardiomyopathy by regulating PHB2-dependent mitochondrial biogenesis.Int J Med Sci. 2024 Apr 8;21(6):983-993. doi: 10.7150/ijms.94577. eCollection 2024. Int J Med Sci. 2024. PMID: 38774750 Free PMC article.
-
Phosphoglycerate mutase 5 exacerbates alcoholic cardiomyopathy in male mice by inducing prohibitin-2 dephosphorylation and impairing mitochondrial quality control.Clin Transl Med. 2024 Aug;14(8):e1806. doi: 10.1002/ctm2.1806. Clin Transl Med. 2024. PMID: 39143739 Free PMC article.
-
PKM2 interacts with and phosphorylates PHB2 to sustain mitochondrial quality control against septic cerebral-cardiac injury.Int J Med Sci. 2024 Jan 21;21(4):633-643. doi: 10.7150/ijms.92367. eCollection 2024. Int J Med Sci. 2024. PMID: 38464826 Free PMC article.
-
Pyruvate Kinase M2: A Potential Regulator of Cardiac Injury Through Glycolytic and Non-glycolytic Pathways.J Cardiovasc Pharmacol. 2024 Jul 1;84(1):1-9. doi: 10.1097/FJC.0000000000001568. J Cardiovasc Pharmacol. 2024. PMID: 38560918 Free PMC article. Review.
-
Mitochondrial autophagy in cardiomyopathy.Curr Opin Genet Dev. 2016 Jun;38:8-15. doi: 10.1016/j.gde.2016.02.006. Epub 2016 Mar 19. Curr Opin Genet Dev. 2016. PMID: 27003723 Free PMC article. Review.
Cited by
-
VSTM2L protects prostate cancer cells against ferroptosis via inhibiting VDAC1 oligomerization and maintaining mitochondria homeostasis.Nat Commun. 2025 Jan 29;16(1):1160. doi: 10.1038/s41467-025-56494-6. Nat Commun. 2025. PMID: 39880844 Free PMC article.
-
Sepsis-induced changes in pyruvate metabolism: insights and potential therapeutic approaches.EMBO Mol Med. 2024 Nov;16(11):2678-2698. doi: 10.1038/s44321-024-00155-6. Epub 2024 Oct 28. EMBO Mol Med. 2024. PMID: 39468303 Free PMC article. Review.
-
Exploiting Mitochondria by Triggering a Faulty Unfolded Protein Response Leads to Effective Cardioprotection.Int J Med Sci. 2025 Jan 1;22(1):188-196. doi: 10.7150/ijms.100523. eCollection 2025. Int J Med Sci. 2025. PMID: 39744160 Free PMC article.
References
-
- Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol. 2019;130:160–169. doi: 10.1016/j.yjmcc.2019.04.006. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

