Secreted antigen A peptidoglycan hydrolase is essential for Enterococcus faecium cell separation and priming of immune checkpoint inhibitor therapy
- PMID: 38857064
- PMCID: PMC11164530
- DOI: 10.7554/eLife.95297
Secreted antigen A peptidoglycan hydrolase is essential for Enterococcus faecium cell separation and priming of immune checkpoint inhibitor therapy
Abstract
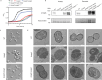
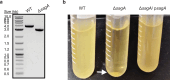
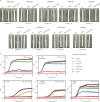
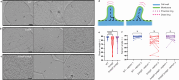
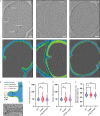
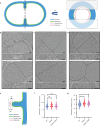
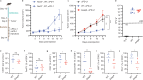
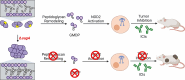
Enterococcus faecium is a microbiota species in humans that can modulate host immunity (Griffin and Hang, 2022), but has also acquired antibiotic resistance and is a major cause of hospital-associated infections (Van Tyne and Gilmore, 2014). Notably, diverse strains of E. faecium produce SagA, a highly conserved peptidoglycan hydrolase that is sufficient to promote intestinal immunity (Rangan et al., 2016; Pedicord et al., 2016; Kim et al., 2019) and immune checkpoint inhibitor antitumor activity (Griffin et al., 2021). However, the functions of SagA in E. faecium were unknown. Here, we report that deletion of sagA impaired E. faecium growth and resulted in bulged and clustered enterococci due to defective peptidoglycan cleavage and cell separation. Moreover, ΔsagA showed increased antibiotic sensitivity, yielded lower levels of active muropeptides, displayed reduced activation of the peptidoglycan pattern-recognition receptor NOD2, and failed to promote cancer immunotherapy. Importantly, the plasmid-based expression of SagA, but not its catalytically inactive mutant, restored ΔsagA growth, production of active muropeptides, and NOD2 activation. SagA is, therefore, essential for E. faecium growth, stress resistance, and activation of host immunity.
Keywords: Enterococcus faecium; NOD2; cancer immunotherapy; immunology; inflammation; mouse.
© 2024, Klupt, Fam, Zhang et al.
Conflict of interest statement
SK, KF, XZ, PC, AM, TB, DG, DP No competing interests declared, HH has filed patent applications (PCT/US2016/028836, PCT/US2020/019038) for the commercial use of SagA-bacteria to improve intestinal immunity and checkpoint blockade immunotherapy, which has been licensed by Rise Therapeutics for probiotic development
Figures

Update of
-
Secreted antigen A peptidoglycan hydrolase is essential for Enterococcus faecium cell separation and priming of immune checkpoint inhibitor therapy.bioRxiv [Preprint]. 2024 Apr 12:2023.11.19.567738. doi: 10.1101/2023.11.19.567738. bioRxiv. 2024. Update in: Elife. 2024 Jun 10;13:RP95297. doi: 10.7554/eLife.95297. PMID: 38014356 Free PMC article. Updated. Preprint.
References
-
- Barnett MPG, McNabb WC, Cookson AL, Zhu S, Davy M, Knoch B, Nones K, Hodgkinson AJ, Roy NC. Changes in colon gene expression associated with increased colon inflammation in interleukin-10 gene-deficient mice inoculated with Enterococcus species. BMC Immunology. 2010;11:39. doi: 10.1186/1471-2172-11-39. - DOI - PMC - PubMed
-
- Belloso Daza MV, Cortimiglia C, Bassi D, Cocconcelli PS. Genome-based studies indicate that the Enterococcus faecium Clade B strains belong to Enterococcus lactis species and lack of the hospital infection associated markers. International Journal of Systematic and Evolutionary Microbiology. 2021;71:004948. doi: 10.1099/ijsem.0.004948. - DOI - PubMed
-
- Belloso Daza MV, Almeida-Santos AC, Novais C, Read A, Alves V, Cocconcelli PS, Freitas AR, Peixe L. Distinction between Enterococcus faecium and Enterococcus lactis by a gluP PCR-Based Assay for Accurate Identification and Diagnostics. Microbiology Spectrum. 2022;10:e0326822. doi: 10.1128/spectrum.03268-22. - DOI - PMC - PubMed
-
- Canfield GS, Chatterjee A, Espinosa J, Mangalea MR, Sheriff EK, Keidan M, McBride SW, McCollister BD, Hang HC, Duerkop BA. Lytic bacteriophages facilitate antibiotic sensitization of Enterococcus faecium. Antimicrobial Agents and Chemotherapy. 2023;65:e00143-21. doi: 10.1128/AAC.00143-21. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

