Subcutaneous Versus Intravenous Amivantamab, Both in Combination With Lazertinib, in Refractory Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer: Primary Results From the Phase III PALOMA-3 Study
- PMID: 38857463
- PMCID: PMC11469630
- DOI: 10.1200/JCO.24.01001
Subcutaneous Versus Intravenous Amivantamab, Both in Combination With Lazertinib, in Refractory Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer: Primary Results From the Phase III PALOMA-3 Study
Abstract
Purpose: Phase III studies of intravenous amivantamab demonstrated efficacy across epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer (NSCLC). A subcutaneous formulation could improve tolerability and reduce administration time while maintaining efficacy.
Patients and methods: Patients with EGFR-mutated advanced NSCLC who progressed after osimertinib and platinum-based chemotherapy were randomly assigned 1:1 to receive subcutaneous or intravenous amivantamab, both combined with lazertinib. Coprimary pharmacokinetic noninferiority end points were trough concentrations (Ctrough; on cycle-2-day-1 or cycle-4-day-1) and cycle-2 area under the curve (AUCD1-D15). Key secondary end points were objective response rate (ORR) and progression-free survival (PFS). Overall survival (OS) was a predefined exploratory end point.
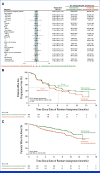
Results: Overall, 418 patients underwent random assignment (subcutaneous group, n = 206; intravenous group, n = 212). Geometric mean ratios of Ctrough for subcutaneous to intravenous amivantamab were 1.15 (90% CI, 1.04 to 1.26) at cycle-2-day-1 and 1.42 (90% CI, 1.27 to 1.61) at cycle-4-day-1; the cycle-2 AUCD1-D15 was 1.03 (90% CI, 0.98 to 1.09). ORR was 30% in the subcutaneous and 33% in the intravenous group; median PFS was 6.1 and 4.3 months, respectively. OS was significantly longer in the subcutaneous versus intravenous group (hazard ratio for death, 0.62; 95% CI, 0.42 to 0.92; nominal P = .02). Fewer patients in the subcutaneous group experienced infusion-related reactions (IRRs; 13% v 66%) and venous thromboembolism (9% v 14%) versus the intravenous group. Median administration time for the first infusion was reduced to 4.8 minutes (range, 0-18) for subcutaneous amivantamab and to 5 hours (range, 0.2-9.9) for intravenous amivantamab. During cycle-1-day-1, 85% and 52% of patients in the subcutaneous and intravenous groups, respectively, considered treatment convenient; the end-of-treatment rates were 85% and 35%, respectively.
Conclusion: Subcutaneous amivantamab-lazertinib demonstrated noninferiority to intravenous amivantamab-lazertinib, offering a consistent safety profile with reduced IRRs, increased convenience, and prolonged survival.
Trial registration: ClinicalTrials.gov NCT05388669.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures









References
-
- Moores SL, Chiu ML, Bushey BS, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76:3942–3953. - PubMed
-
- Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther. 2020;19:2044–2056. - PubMed
-
- Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10:1194–1209. - PubMed
-
- Cho BC, Simi A, Sabari J, et al. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer. 2023;24:89–97. - PubMed
-
- Besse B, Baik CS, Marmarelis ME, et al. Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR-mutated NSCLC in the post-osimertinib setting: Analysis of tissue IHC and ctDNA NGS. J Clin Oncol. 2023;41:9013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

