Current Induced Spin-Polarization in Chiral Molecules
- PMID: 38857512
- PMCID: PMC11194818
- DOI: 10.1021/acs.jpclett.4c01362
Current Induced Spin-Polarization in Chiral Molecules
Abstract
The inverse spin-galvanic effect or current-induced spin-polarization is mainly associated with interfaces between different layers in semiconducting heterostructures, surfaces of metals, and bulk semiconducting materials. Here, we theoretically predict that the inverse spin-galvanic effect should also be present in chiral molecules, as a result of the chiral induced spin selectivity effect. As proof-of-principle, we calculate the nonequilibrium properties of a model system that previously has been successfully used to explain a multitude of aspects related to the chiral induced spin selectivity effect. Here we show that current driven spin-polarization in a chiral molecule gives rise to a magnetic moment that is sensitive to external magnet field. The chiral molecule then behaves like a soft ferromagnet. This, in turn, suggests that magnetic permeability measurement in otherwise nonmagnetic systems may be used noninvasively to detect the presence of spin-polarized currents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
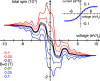
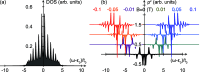
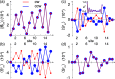
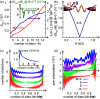
 and a voltage bias of 1 V. (a) Induced
magnetic moment SMol (red) and corresponding
longitudinal spin susceptibility dSMol/dBz (purple) and (b)
spin-susceptibility as a function of polar angle (θ) orientation
of the magnetic field relative to the length direction of the molecule,
of length 4 × 8. (c) Site resolved charge distributions for M = 4, 6, 9, and 14, and (d) corresponding site-resolved
spin-polarization. The insets in panels (a), (b), and (c) show the
transverse spin susceptibility dSMol/dBx, a schematic of the setup,
and the charge current, respectively. The plots in panels (c) and
(d) are offset for clarity. Other parameters are as shown in Figure 1.
and a voltage bias of 1 V. (a) Induced
magnetic moment SMol (red) and corresponding
longitudinal spin susceptibility dSMol/dBz (purple) and (b)
spin-susceptibility as a function of polar angle (θ) orientation
of the magnetic field relative to the length direction of the molecule,
of length 4 × 8. (c) Site resolved charge distributions for M = 4, 6, 9, and 14, and (d) corresponding site-resolved
spin-polarization. The insets in panels (a), (b), and (c) show the
transverse spin susceptibility dSMol/dBx, a schematic of the setup,
and the charge current, respectively. The plots in panels (c) and
(d) are offset for clarity. Other parameters are as shown in Figure 1.Similar articles
-
Charge Redistribution and Spin Polarization Driven by Correlation Induced Electron Exchange in Chiral Molecules.Nano Lett. 2021 Apr 14;21(7):3026-3032. doi: 10.1021/acs.nanolett.1c00183. Epub 2021 Mar 24. Nano Lett. 2021. PMID: 33759530 Free PMC article.
-
Chirality and Spin: A Different Perspective on Enantioselective Interactions.Chimia (Aarau). 2018 Jun 27;72(6):394-398. doi: 10.2533/chimia.2018.394. Chimia (Aarau). 2018. PMID: 29941075
-
Spin-Dependent Transport through Chiral Molecules Studied by Spin-Dependent Electrochemistry.Acc Chem Res. 2016 Nov 15;49(11):2560-2568. doi: 10.1021/acs.accounts.6b00446. Epub 2016 Oct 24. Acc Chem Res. 2016. PMID: 27797176 Free PMC article.
-
Spin Polarization in Transport Studies of Chirality-Induced Spin Selectivity.ACS Nano. 2023 Oct 24;17(20):19502-19507. doi: 10.1021/acsnano.3c06133. Epub 2023 Oct 4. ACS Nano. 2023. PMID: 37793070 Review.
-
Chiral-Molecule-Based Spintronic Devices.Small. 2022 Aug;18(32):e2203015. doi: 10.1002/smll.202203015. Epub 2022 Jul 14. Small. 2022. PMID: 35836101 Review.
Cited by
-
Chiral Phonons Enhance Ferromagnetism.J Phys Chem Lett. 2025 Feb 27;16(8):2001-2007. doi: 10.1021/acs.jpclett.5c00304. Epub 2025 Feb 18. J Phys Chem Lett. 2025. PMID: 39965120 Free PMC article.
-
Chiral Induced Spin Polarized Electron Current: Origin of the Chiral Induced Spin Selectivity Effect.J Phys Chem Lett. 2025 May 1;16(17):4346-4353. doi: 10.1021/acs.jpclett.5c00104. Epub 2025 Apr 24. J Phys Chem Lett. 2025. PMID: 40270227 Free PMC article.
-
Chirality Assisted Triplet Electron Pairing.J Phys Chem Lett. 2025 Feb 13;16(6):1629-1633. doi: 10.1021/acs.jpclett.4c03734. Epub 2025 Feb 5. J Phys Chem Lett. 2025. PMID: 39907703 Free PMC article.
References
-
- Rees D. J. Chem. Educ. 1983, 60, 289.10.1021/ed060p289. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

