The life cycle of a capillary: Mechanisms of angiogenesis and rarefaction in microvascular physiology and pathologies
- PMID: 38857638
- PMCID: PMC12051481
- DOI: 10.1016/j.vph.2024.107393
The life cycle of a capillary: Mechanisms of angiogenesis and rarefaction in microvascular physiology and pathologies
Abstract
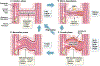
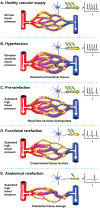
Capillaries are the smallest blood vessels (<10 μm in diameter) in the body and their walls are lined by endothelial cells. These microvessels play a crucial role in nutrient and gas exchange between blood and tissues. Capillary endothelial cells also produce vasoactive molecules and initiate the electrical signals that underlie functional hyperemia and neurovascular coupling. Accordingly, capillary function and density are critical for all cell types to match blood flow to cellular activity. This begins with the process of angiogenesis, when new capillary blood vessels emerge from pre-existing vessels, and ends with rarefaction, the loss of these microvascular structures. This review explores the mechanisms behind these processes, emphasizing their roles in various microvascular diseases and their impact on surrounding cells in health and disease. We discuss recent work on the mechanisms controlling endothelial cell proliferation, migration, and tube formation that underlie angiogenesis under physiological and pathological conditions. The mechanisms underlying functional and anatomical rarefaction and the role of pericytes in this process are also discussed. Based on this work, a model is proposed in which the balance of angiogenic and rarefaction signaling pathways in a particular tissue match microvascular density to the metabolic demands of the surrounding cells. This negative feedback loop becomes disrupted during microvascular rarefaction: angiogenic mechanisms are blunted, reactive oxygen species accumulate, capillary function declines and eventually, capillaries disappear. This, we propose, forms the foundation of the reciprocal relationship between vascular density, blood flow, and metabolic needs and functionality of nearby cells.
Keywords: Angiogenesis; Arteriogenesis; Heart failure with preserved ejection fraction; Hypertension; Microvascular rarefaction; Pericytes; Small vessel disease; Vascular dementia.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

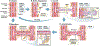


Similar articles
-
Systemic microvascular rarefaction is correlated with dysfunction of late endothelial progenitor cells in mild hypertension: a substudy of EXCAVATION-CHN1.J Transl Med. 2019 Nov 12;17(1):368. doi: 10.1186/s12967-019-2108-8. J Transl Med. 2019. PMID: 31718666 Free PMC article. Clinical Trial.
-
Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis.Am J Pathol. 2011 Feb;178(2):911-23. doi: 10.1016/j.ajpath.2010.10.012. Am J Pathol. 2011. PMID: 21281822 Free PMC article.
-
Impaired skeletal muscle performance as a consequence of random functional capillary rarefaction can be restored with overload-dependent angiogenesis.J Physiol. 2020 Mar;598(6):1187-1203. doi: 10.1113/JP278975. Epub 2020 Feb 26. J Physiol. 2020. PMID: 32012275 Free PMC article.
-
Metabolic Syndrome and Cardiac Vessel Remodeling Associated with Vessel Rarefaction: A Possible Underlying Mechanism May Result from a Poor Angiogenic Response to Altered VEGF Signaling Pathways.J Vasc Res. 2024;61(4):151-159. doi: 10.1159/000538361. Epub 2024 Apr 12. J Vasc Res. 2024. PMID: 38615659 Review.
-
Microvascular cells: A special focus on heterogeneity of pericytes in diabetes associated complications.Int J Biochem Cell Biol. 2021 May;134:105971. doi: 10.1016/j.biocel.2021.105971. Epub 2021 Mar 26. Int J Biochem Cell Biol. 2021. PMID: 33775914 Review.
Cited by
-
Glucose Metabolism Reprogramming of Vascular Endothelial Cells and Its Implication in Development of Atherosclerosis.Rev Cardiovasc Med. 2024 Nov 22;25(11):423. doi: 10.31083/j.rcm2511423. eCollection 2024 Nov. Rev Cardiovasc Med. 2024. PMID: 39618869 Free PMC article. Review.
-
Melatonin Promotes Cerebral Angiogenesis in Ischemic Mice via BMP6/Smad1/5/9 Pathway.Mol Neurobiol. 2025 Sep;62(9):11362-11381. doi: 10.1007/s12035-025-04969-4. Epub 2025 Apr 25. Mol Neurobiol. 2025. PMID: 40274709 Free PMC article.
-
Increased luminal pressure in brain capillaries drives TRPC3-dependent depolarization and constriction of transitional pericytes.Sci Signal. 2025 Apr 29;18(884):eads1903. doi: 10.1126/scisignal.ads1903. Epub 2025 Apr 29. Sci Signal. 2025. PMID: 40299956 Free PMC article.
-
Isolated human adipose microvessels retain native microvessel structure and recapitulate sprouting angiogenesis.Angiogenesis. 2025 Mar 6;28(2):20. doi: 10.1007/s10456-025-09972-w. Angiogenesis. 2025. PMID: 40048000
-
Skin microcirculation and hypertension: is there a connection?J Bras Nefrol. 2025 Jul-Sep;47(3):e202440192. doi: 10.1590/2175-8239-JBN-2024-0192en. J Bras Nefrol. 2025. PMID: 40456017 Free PMC article. Review.
References
-
- Potente M, Makinen T, Vascular heterogeneity and specialization in development and disease, Nat. Rev. Mol. Cell Biol. 18 (8) (2017) 477–494. - PubMed
-
- Spadaccio C, Nenna A, Rose D, Piccirillo F, Nusca A, Grigioni F, Chello M, Vlahakes GJ, The role of angiogenesis and Arteriogenesis in myocardial infarction and coronary revascularization, J. Cardiovasc. Transl. Res 15 (5) (2022) 1024–1048. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

