G272V and P301L Mutations Induce Isoform Specific Tau Mislocalization to Dendritic Spines and Synaptic Dysfunctions in Cellular Models of 3R and 4R Tau Frontotemporal Dementia
- PMID: 38858079
- PMCID: PMC11236579
- DOI: 10.1523/JNEUROSCI.1215-23.2024
G272V and P301L Mutations Induce Isoform Specific Tau Mislocalization to Dendritic Spines and Synaptic Dysfunctions in Cellular Models of 3R and 4R Tau Frontotemporal Dementia
Abstract
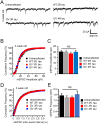
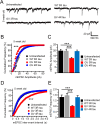
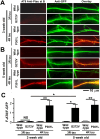
Tau pathologies are detected in the brains of some of the most common neurodegenerative diseases including Alzheimer's disease (AD), Lewy body dementia (LBD), chronic traumatic encephalopathy (CTE), and frontotemporal dementia (FTD). Tau proteins are expressed in six isoforms with either three or four microtubule-binding repeats (3R tau or 4R tau) due to alternative RNA splicing. AD, LBD, and CTE brains contain pathological deposits of both 3R and 4R tau. FTD patients can exhibit either 4R tau pathologies in most cases or 3R tau pathologies less commonly in Pick's disease, which is a subfamily of FTD. Here, we report the isoform-specific roles of tau in FTD. The P301L mutation, linked to familial 4R tau FTD, induces mislocalization of 4R tau to dendritic spines in primary hippocampal cultures that were prepared from neonatal rat pups of both sexes. Contrastingly, the G272V mutation, linked to familial Pick's disease, induces phosphorylation-dependent mislocalization of 3R tau but not 4R tau proteins to dendritic spines. The overexpression of G272V 3R tau but not 4R tau proteins leads to the reduction of dendritic spine density and suppression of mEPSCs in 5-week-old primary rat hippocampal cultures. The decrease in mEPSC amplitude caused by G272V 3R tau is dynamin-dependent whereas that caused by P301L 4R tau is dynamin-independent, indicating that the two tau isoforms activate different signaling pathways responsible for excitatory synaptic dysfunction. Our 3R and 4R tau studies here will shed new light on diverse mechanisms underlying FTD, AD, LBD, and CTE.
Keywords: AMPA receptors; Alzheimer's disease; dendritic spines; dynamin; frontotemporal dementia; tau mislocalization.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
Development of a novel tau propagation mouse model endogenously expressing 3 and 4 repeat tau isoforms.Brain. 2022 Mar 29;145(1):349-361. doi: 10.1093/brain/awab289. Brain. 2022. PMID: 34515757
-
Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer's disease.Acta Neuropathol Commun. 2021 May 12;9(1):86. doi: 10.1186/s40478-021-01189-4. Acta Neuropathol Commun. 2021. PMID: 33980303 Free PMC article.
-
Tau PET Imaging with [18F]PM-PBB3 in Frontotemporal Dementia with MAPT Mutation.J Alzheimers Dis. 2020;76(1):149-157. doi: 10.3233/JAD-200287. J Alzheimers Dis. 2020. PMID: 32444551
-
[The genetics of dementias. Part 1: Molecular basis of frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)].Postepy Hig Med Dosw (Online). 2009 Jun 15;63:278-86. Postepy Hig Med Dosw (Online). 2009. PMID: 19535823 Review. Polish.
-
The Role of Tau Proteoforms in Health and Disease.Mol Neurobiol. 2023 Sep;60(9):5155-5166. doi: 10.1007/s12035-023-03387-8. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266762 Review.
Cited by
-
Differentiation of SH-SY5Y Cells into Cortical Neuron-like Cells for Tauopathy Modeling and Seeding Assays.Mol Neurobiol. 2025 Jun 4. doi: 10.1007/s12035-025-05100-3. Online ahead of print. Mol Neurobiol. 2025. PMID: 40467940
-
Temporal transcriptomic changes in the THY-Tau22 mouse model of tauopathy display cell type- and sex-specific differences.Acta Neuropathol Commun. 2025 May 7;13(1):93. doi: 10.1186/s40478-025-02013-z. Acta Neuropathol Commun. 2025. PMID: 40336141 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
