The nucleolar phase of signal recognition particle assembly
- PMID: 38858088
- PMCID: PMC11165425
- DOI: 10.26508/lsa.202402614
The nucleolar phase of signal recognition particle assembly
Abstract
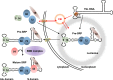
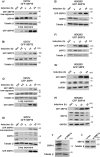
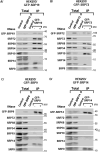
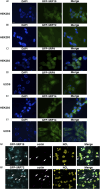
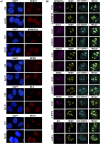
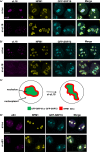
The signal recognition particle is essential for targeting transmembrane and secreted proteins to the endoplasmic reticulum. Remarkably, because they work together in the cytoplasm, the SRP and ribosomes are assembled in the same biomolecular condensate: the nucleolus. How important is the nucleolus for SRP assembly is not known. Using quantitative proteomics, we have investigated the interactomes of SRP components. We reveal that SRP proteins are associated with scores of nucleolar proteins important for ribosome biogenesis and nucleolar structure. Having monitored the subcellular distribution of SRP proteins upon controlled nucleolar disruption, we conclude that an intact organelle is required for their proper localization. Lastly, we have detected two SRP proteins in Cajal bodies, which indicates that previously undocumented steps of SRP assembly may occur in these bodies. This work highlights the importance of a structurally and functionally intact nucleolus for efficient SRP production and suggests that the biogenesis of SRP and ribosomes may be coordinated in the nucleolus by common assembly factors.
© 2024 Issa et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, Azzag K, Robert MC, Ahmad Y, Neel H, et al. (2010. a) HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell 39: 912–924. 10.1016/j.molcel.2010.08.023 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
