Efficacy and safety of tirofiban in patients with acute branch atheromatous disease-related stroke (BRANT): a protocol for a randomised controlled trial
- PMID: 38858147
- PMCID: PMC11168161
- DOI: 10.1136/bmjopen-2023-082141
Efficacy and safety of tirofiban in patients with acute branch atheromatous disease-related stroke (BRANT): a protocol for a randomised controlled trial
Abstract
Introduction: Branch atheromatous disease (BAD)-related stroke is increasingly becoming a clinical entity and prone to early neurological deterioration (END) and poor prognosis. There are no effective regimens to reduce the disability caused by BAD-related stroke in acute phase. Recent studies have indicated the efficacy of tirofiban in acute ischaemic stroke; however, its efficacy has not been validated in patients with BAD-related stroke. Thus, we aim to test whether intravenous tirofiban initiated within 48 hours after the onset would improve the functional outcome in patients with acute BAD-related stroke, in comparison with the standard antiplatelet therapy based on the current guideline.
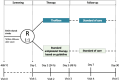
Methods and analysis: BRANT is a multicentre, randomised, open-label, blinded endpoint, parallel-controlled, phase III trial conducted in 21 hospitals in China. Participants aged 18-75 years with acute BAD-related stroke within 48 hours after the stroke onset are randomised in a 1:1 ratio to the tirofiban or control group. The treatment period is 48 hours in both groups. The primary outcome is the excellent functional outcome (modified Rankin Scale Score: 0-1) at 90 days. The secondary outcomes include END, major bleeding, stroke, death, functional status, serious adverse events and change in bleeding-related markers. Assuming the rates of the primary outcome to be 74% in the tirofiban group and 62% in the control group, a total of 516 participants are needed for 0.8 power (two-sided 0.05 alpha).
Ethics and dissemination: BRANT study has been approved by the Ethics Committee of the Peking Union Medical College Hospital (I-23PJ1242). Written informed consent is required for all the patients before enrolment. The results of the study will be published in a peer-reviewed journal.
Trial registration number: ClinicalTrials.gov (NCT06037889).
Keywords: Prognosis; Stroke; THERAPEUTICS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effects of Tirofiban on Neurological Deterioration in Patients With Acute Ischemic Stroke: A Randomized Clinical Trial.JAMA Neurol. 2024 Jun 1;81(6):594-602. doi: 10.1001/jamaneurol.2024.0868. JAMA Neurol. 2024. PMID: 38648030 Free PMC article. Clinical Trial.
-
Early tirofiban administration after intravenous thrombolysis in acute ischemic stroke (ADVENT): Study protocol of a multicenter, randomized, double-blind, placebo-controlled clinical trial.Eur Stroke J. 2024 Jun;9(2):510-514. doi: 10.1177/23969873231225069. Epub 2024 Jan 9. Eur Stroke J. 2024. PMID: 38196129 Free PMC article.
-
Advancing stroke safety and efficacy through early tirofiban administration after intravenous thrombolysis: The multicenter, randomized, placebo-controlled, double-blind ASSET IT trial protocol.Int J Stroke. 2025 Mar;20(3):373-377. doi: 10.1177/17474930241299666. Epub 2024 Nov 20. Int J Stroke. 2025. PMID: 39501470
-
Tirofiban Combination Therapy for Acute Ischemic Stroke: A Systematic Review and Meta-Analysis.Brain Behav. 2025 Jun;15(6):e70508. doi: 10.1002/brb3.70508. Brain Behav. 2025. PMID: 40495442 Free PMC article.
-
The Application of Tirofiban in the Endovascular Treatment of Acute Ischemic Stroke: A Meta-Analysis.Cerebrovasc Dis. 2021;50(2):121-131. doi: 10.1159/000512601. Epub 2021 Jan 5. Cerebrovasc Dis. 2021. PMID: 33401276 Review.
Cited by
-
Acute cerebral small vessel disease: Classification, mechanism, and therapeutic implications.Chin Med J (Engl). 2024 Nov 5;137(21):2561-2563. doi: 10.1097/CM9.0000000000003304. Epub 2024 Sep 27. Chin Med J (Engl). 2024. PMID: 39329290 Free PMC article. No abstract available.
-
Tirofiban in Acute Ischemic Stroke: Mechanistic Rationale, Clinical Advances, and Emerging Therapeutic Strategies.Drugs. 2025 Aug 22. doi: 10.1007/s40265-025-02222-9. Online ahead of print. Drugs. 2025. PMID: 40847265 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials