Faecal microbiota transplantation (FMT) in Norwegian outpatients with mild to severe myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): protocol for a 12-month randomised double-blind placebo-controlled trial
- PMID: 38858151
- PMCID: PMC11168185
- DOI: 10.1136/bmjopen-2023-073275
Faecal microbiota transplantation (FMT) in Norwegian outpatients with mild to severe myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): protocol for a 12-month randomised double-blind placebo-controlled trial
Abstract
Introduction: The observed alteration of the intestinal microbiota in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and the effect of transferring a healthy gut flora from a faecal donor using a faecal microbiota transplantation (FMT) will be explored in this trial.
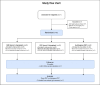
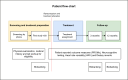
Methods and analysis: This is a protocol for a randomised, double-blind, placebo-controlled, parallel-group, single-centre trial, with 12 months follow-up. 80 participants will be included and randomised (1:1:2) to either donor FMT (from two different donors) or placebo (autologous FMT). Participants will be included by the International Clinical Criteria for ME/CFS. The clinical measures of ME/CFS and disease activity include Modified DePaul Questionnaire, Fatigue Severity Scale (FSS), Hospital Anxiety and Depression Scale (HADS), 36-Item Short Form Health Survey (SF-36), ROMA IV criteria, Food Frequency Questionnaire, Repeatable Battery for the Assessment of Neuropsychological Status, heart rate variability testing and reports on the use of antibiotics and food supplements, as well as biobanking of blood, urine and faeces.The primary endpoint is proportion with treatment success in FSS score in donor versus autologous FMT group 3 months after treatment. Treatment success is defined as an FSS improvement of more than 1.2 points from baseline at 3 months after treatment. Adverse events will be registered throughout the study.
Ethics and dissemination: The Regional Committee for Medical Research Ethics Northern Norway has approved the study. The study has commenced in May 2019. Findings will be disseminated in international peer-reviewed journal(s), submitted to relevant conferences, and trial participants will be informed via phone calls.
Trial registration number: NCT03691987.
Keywords: Clinical Trial; Fatigue; Gastroenterology; Irritable Bowel Syndrome; MICROBIOLOGY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Estévez-López F, Mudie K, Wang-Steverding X, et al. Systematic review of the Epidemiological burden of Myalgic Encephalomyelitis/chronic fatigue syndrome across Europe: Current evidence and EUROMENE research recommendations for epidemiology. J Clin Med 2020;9:1557. 10.3390/jcm9051557 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical