ERAS for Ambulatory TURBT: Enhancing Bladder Cancer Care (EMBRACE) randomised controlled trial protocol
- PMID: 38858157
- PMCID: PMC11168167
- DOI: 10.1136/bmjopen-2023-076763
ERAS for Ambulatory TURBT: Enhancing Bladder Cancer Care (EMBRACE) randomised controlled trial protocol
Abstract
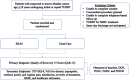
Introduction: Transurethral resection of bladder tumour (TURBT) is one of the more common procedures performed by urologists. It is often described as an 'incision-free' and 'well-tolerated' operation. However, many patients experience distress and discomfort with the procedure. Substantial opportunity exists to improve the TURBT experience. An enhanced recovery after surgery (ERAS) protocol designed by patients with bladder cancer and their providers has been developed.
Methods and analysis: This is a single-centre, randomised controlled trial to investigate the effectiveness of an ERAS protocol compared with usual care in patients with bladder cancer undergoing ambulatory TURBT. The ERAS protocol is composed of preoperative, intraoperative and postoperative components designed to optimise each phase of perioperative care. 100 patients with suspected or known bladder cancer aged ≥18 years undergoing initial or repeat ambulatory TURBT will be enrolled. The change in Quality of Recovery 15 score, a measure of the quality of recovery, between the day of surgery and postoperative day 1 will be compared between the ERAS and control groups.
Ethics and dissemination: The trial has been approved by the Johns Hopkins Institutional Review Board #00392063. Participants will provide informed consent to participate before taking part in the study. Results will be reported in a separate publication.
Trial registration number: NCT05905276.
Keywords: Adult oncology; Bladder disorders; Patient Reported Outcome Measures; UROLOGY; Urological tumours.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Best Practices to Optimise Quality and Outcomes of Transurethral Resection of Bladder Tumours.Eur Urol Oncol. 2021 Feb;4(1):12-19. doi: 10.1016/j.euo.2020.06.010. Epub 2020 Jul 16. Eur Urol Oncol. 2021. PMID: 32684515 Review.
-
Efficacy and safety of transurethral resection of bladder tumour combined with chemotherapy and immunotherapy in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer: a protocol for a randomized controlled trial.BMC Cancer. 2023 Apr 6;23(1):320. doi: 10.1186/s12885-023-10798-2. BMC Cancer. 2023. PMID: 37024824 Free PMC article.
-
A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer.World J Urol. 2018 Feb;36(2):215-220. doi: 10.1007/s00345-017-2109-2. Epub 2017 Nov 7. World J Urol. 2018. PMID: 29116394 Clinical Trial.
-
Fluorescent cystoscopy-assisted en bloc transurethral resection versus conventional transurethral resection in patients with non-muscle invasive bladder cancer: study protocol of a prospective, open-label, randomized control trial (the FLEBER study).Trials. 2021 Feb 12;22(1):136. doi: 10.1186/s13063-021-05094-y. Trials. 2021. PMID: 33579327 Free PMC article.
-
Impact of enhanced recovery after surgery protocols versus standard of care on perioperative outcomes of radical cystectomy: a systematic review and meta-analysis of comparative studies.Minerva Urol Nefrol. 2019 Aug;71(4):309-323. doi: 10.23736/S0393-2249.19.03376-9. Epub 2019 Jun 21. Minerva Urol Nefrol. 2019. PMID: 31241271
Cited by
-
Optimizing Pharmacotherapy During Implementation of Enhanced Recovery After Surgery (ERAS) in Ambulatory Urologic Oncology Surgery: Narrative Review.Cancers (Basel). 2025 Feb 11;17(4):614. doi: 10.3390/cancers17040614. Cancers (Basel). 2025. PMID: 40002209 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical