In situ brain tumor detection using a Raman spectroscopy system-results of a multicenter study
- PMID: 38858389
- PMCID: PMC11164901
- DOI: 10.1038/s41598-024-62543-9
In situ brain tumor detection using a Raman spectroscopy system-results of a multicenter study
Abstract
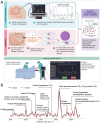
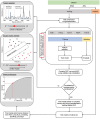
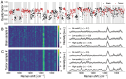
Safe and effective brain tumor surgery aims to remove tumor tissue, not non-tumoral brain. This is a challenge since tumor cells are often not visually distinguishable from peritumoral brain during surgery. To address this, we conducted a multicenter study testing whether the Sentry System could distinguish the three most common types of brain tumors from brain tissue in a label-free manner. The Sentry System is a new real time, in situ brain tumor detection device that merges Raman spectroscopy with machine learning tissue classifiers. Nine hundred and seventy-six in situ spectroscopy measurements and colocalized tissue specimens were acquired from 67 patients undergoing surgery for glioblastoma, brain metastases, or meningioma to assess tumor classification. The device achieved diagnostic accuracies of 91% for glioblastoma, 97% for brain metastases, and 96% for meningiomas. These data show that the Sentry System discriminated tumor containing tissue from non-tumoral brain in real time and prior to resection.
© 2024. The Author(s).
Conflict of interest statement
Frederic Leblond and Kevin Petrecca are co-founders of Reveal Surgical. Authors listed as "Reveal Surgical" worked within the company Reveal Surgical. However, none were involved in data analysis or interpretation. Costantino Hadjipanayis is paid consultant for Synaptive Medical, Stryker Corporation, and Hemerion.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

