Aberrant ER-mitochondria communication is a common pathomechanism in mitochondrial disease
- PMID: 38858390
- PMCID: PMC11164949
- DOI: 10.1038/s41419-024-06781-9
Aberrant ER-mitochondria communication is a common pathomechanism in mitochondrial disease
Abstract
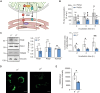
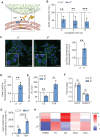
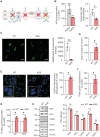
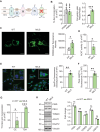
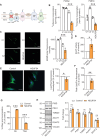
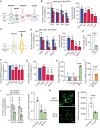
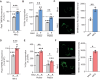
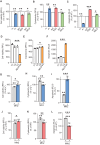
Genetic mutations causing primary mitochondrial disease (i.e those compromising oxidative phosphorylation [OxPhos]) resulting in reduced bioenergetic output display great variability in their clinical features, but the reason for this is unknown. We hypothesized that disruption of the communication between endoplasmic reticulum (ER) and mitochondria at mitochondria-associated ER membranes (MAM) might play a role in this variability. To test this, we assayed MAM function and ER-mitochondrial communication in OxPhos-deficient cells, including cybrids from patients with selected pathogenic mtDNA mutations. Our results show that each of the various mutations studied indeed altered MAM functions, but notably, each disorder presented with a different MAM "signature". We also found that mitochondrial membrane potential is a key driver of ER-mitochondrial connectivity. Moreover, our findings demonstrate that disruption in ER-mitochondrial communication has consequences for cell survivability that go well beyond that of reduced ATP output. The findings of a "MAM-OxPhos" axis, the role of mitochondrial membrane potential in controlling this process, and the contribution of MAM dysfunction to cell death, reveal a new relationship between mitochondria and the rest of the cell, as well as providing new insights into the diagnosis and treatment of these devastating disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease.EMBO J. 2017 Nov 15;36(22):3356-3371. doi: 10.15252/embj.201796797. Epub 2017 Oct 10. EMBO J. 2017. PMID: 29018038 Free PMC article.
-
Mitochondrial Mutation Leads to Cardiomyocyte Hypertrophy by Disruption of Mitochondria-Associated ER Membrane.Cell Prolif. 2025 Jul;58(7):e70002. doi: 10.1111/cpr.70002. Epub 2025 Feb 21. Cell Prolif. 2025. PMID: 39981966 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Smoking cessation for secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2022 Aug 8;8(8):CD014936. doi: 10.1002/14651858.CD014936.pub2. Cochrane Database Syst Rev. 2022. PMID: 35938889 Free PMC article.
Cited by
-
Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell.Biomolecules. 2024 Nov 29;14(12):1534. doi: 10.3390/biom14121534. Biomolecules. 2024. PMID: 39766241 Free PMC article. Review.
-
The AICD interactome: implications in neurodevelopment and neurodegeneration.Biochem Soc Trans. 2024 Dec 19;52(6):2539-2556. doi: 10.1042/BST20241510. Biochem Soc Trans. 2024. PMID: 39670668 Free PMC article. Review.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes in Human Health and Diseases.MedComm (2020). 2025 Jun 27;6(7):e70259. doi: 10.1002/mco2.70259. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40584408 Free PMC article. Review.
-
Modulation of Mitochondria-Endoplasmic Reticulum Contacts (MERCs) by Small Molecules as a New Strategy for Restoring Lipid Metabolism in an Amyotrophic Lateral Sclerosis Model.J Med Chem. 2025 Jan 23;68(2):1179-1194. doi: 10.1021/acs.jmedchem.4c01368. Epub 2025 Jan 8. J Med Chem. 2025. PMID: 39778888 Free PMC article.
-
Recent development of mitochondrial metabolism and dysfunction in osteoarthritis.Front Pharmacol. 2025 Feb 13;16:1538662. doi: 10.3389/fphar.2025.1538662. eCollection 2025. Front Pharmacol. 2025. PMID: 40017603 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

