Antiviral activity of pimecrolimus against dengue virus type 2 infection in vitro and in vivo
- PMID: 38858399
- PMCID: PMC11164929
- DOI: 10.1038/s41598-024-61127-x
Antiviral activity of pimecrolimus against dengue virus type 2 infection in vitro and in vivo
Abstract
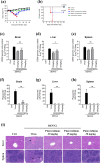
Dengue virus (DENV) infection is a public health concern in several countries and is associated with severe diseases, such as dengue hemorrhagic fever and dengue shock syndrome. DENVs are transmitted to humans via the bites of infected Aedes mosquitoes, and no antiviral therapeutics are currently available. In this work, we aimed to identify antiviral drugs against DENV type 2 (DENV2) infections and selected pimecrolimus as a potential antiviral drug candidate. Pimecrolimus significantly inhibited DENV2-mediated cell death and replication in vitro. We also confirmed a decrease in the number of plaques formed as well as in the envelope protein levels of DENV2. The time-of-addition and course experiments revealed that pimecrolimus inhibited DENV2 infection during the early stages of the virus replication cycle. In an experimental mouse model, orally administered pimecrolimus alleviated body weight loss and lethality caused by DENV2 infection, which we used as readouts of the drug's antiviral potency. Furthermore, pimecrolimus significantly inhibited the DENV2 load and ameliorated focal necrosis in the liver and spleen. Taken together, our in vitro and in vivo findings suggest that pimecrolimus is a promising antiviral drug candidate for the treatment of DENV2 infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Efficacy of geraniin on dengue virus type-2 infected BALB/c mice.Virol J. 2019 Feb 27;16(1):26. doi: 10.1186/s12985-019-1127-7. Virol J. 2019. PMID: 30813954 Free PMC article.
-
Carnosine exhibits significant antiviral activity against Dengue and Zika virus.J Pept Sci. 2019 Aug;25(8):e3196. doi: 10.1002/psc.3196. Epub 2019 Jul 9. J Pept Sci. 2019. PMID: 31290226
-
Tatanan A from the Acorus calamus L. root inhibited dengue virus proliferation and infections.Phytomedicine. 2018 Mar 15;42:258-267. doi: 10.1016/j.phymed.2018.03.018. Epub 2018 Mar 15. Phytomedicine. 2018. PMID: 29655694
-
Antiviral Role of Phenolic Compounds against Dengue Virus: A Review.Biomolecules. 2020 Dec 24;11(1):11. doi: 10.3390/biom11010011. Biomolecules. 2020. PMID: 33374457 Free PMC article. Review.
-
Preclinical Antiviral Testing for Dengue Virus Infection in Mouse Models and Its Association with Clinical Studies.ACS Infect Dis. 2018 Jul 13;4(7):1048-1057. doi: 10.1021/acsinfecdis.8b00054. Epub 2018 Jun 1. ACS Infect Dis. 2018. PMID: 29756760 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

