Multivalent mRNA-DTP vaccines are immunogenic and provide protection from Bordetella pertussis challenge in mice
- PMID: 38858423
- PMCID: PMC11164898
- DOI: 10.1038/s41541-024-00890-4
Multivalent mRNA-DTP vaccines are immunogenic and provide protection from Bordetella pertussis challenge in mice
Abstract
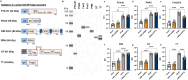
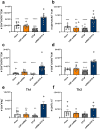
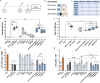
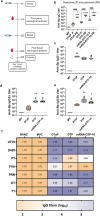
Acellular multivalent vaccines for pertussis (DTaP and Tdap) prevent symptomatic disease and infant mortality, but immunity to Bordetella pertussis infection wanes significantly over time resulting in cyclic epidemics of pertussis. The messenger RNA (mRNA) vaccine platform provides an opportunity to address complex bacterial infections with an adaptable approach providing Th1-biased responses. In this study, immunogenicity and challenge models were used to evaluate the mRNA platform with multivalent vaccine formulations targeting both B. pertussis antigens and diphtheria and tetanus toxoids. Immunization with mRNA formulations were immunogenetic, induced antigen specific antibodies, as well as Th1 T cell responses. Upon challenge with either historical or contemporary B. pertussis strains, 6 and 10 valent mRNA DTP vaccine provided protection equal to that of 1/20th human doses of either DTaP or whole cell pertussis vaccines. mRNA DTP immunized mice were also protected from pertussis toxin challenge as measured by prevention of lymphocytosis and leukocytosis. Collectively these pre-clinical mouse studies illustrate the potential of the mRNA platform for multivalent bacterial pathogen vaccines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Edwards, K. E. & Decker, M. D. In Plotkin’s Vaccines (eds. Plotkin, S. A., OrenStein, W., Offit, P. & Edwards, K. M.) 711–761 (Elsevier, 2018)
-
- CDC & NCIRD. Immunology and Vaccine-Preventable Diseases—Pink Book—Pertussis. (CDC &NCIRD, 2021)
Grants and funding
- R01 AI137155/AI/NIAID NIH HHS/United States
- 5R01AI153250-04/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI153250/AI/NIAID NIH HHS/United States
- P20 GM121322/GM/NIGMS NIH HHS/United States
- P20 GM103434/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

