Evaluating the antibody response elicited by diverse HIV envelope immunogens in the African green monkey (Vervet) model
- PMID: 38858452
- PMCID: PMC11164991
- DOI: 10.1038/s41598-024-63703-7
Evaluating the antibody response elicited by diverse HIV envelope immunogens in the African green monkey (Vervet) model
Abstract
African Green (Vervet) monkeys have been extensively studied to understand the pathogenesis of infectious diseases. Using vervet monkeys as pre-clinical models may be an attractive option for low-resourced areas as they are found abundantly and their maintenance is more cost-effective than bigger primates such as rhesus macaques. We assessed the feasibility of using vervet monkeys as animal models to examine the immunogenicity of HIV envelope trimer immunogens in pre-clinical testing. Three groups of vervet monkeys were subcutaneously immunized with either the BG505 SOSIP.664 trimer, a novel subtype C SOSIP.664 trimer, CAP255, or a combination of BG505, CAP255 and CAP256.SU SOSIP.664 trimers. All groups of vervet monkeys developed robust binding antibodies by the second immunization with the peak antibody response occurring after the third immunization. Similar to binding, antibody dependent cellular phagocytosis was also observed in all the monkeys. While all animals developed potent, heterologous Tier 1 neutralizing antibody responses, autologous neutralization was limited with only half of the animals in each group developing responses to their vaccine-matched pseudovirus. These data suggest that the vervet monkey model may yield distinct antibody responses compared to other models. Further study is required to further determine the utility of this model in HIV immunization studies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


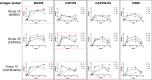
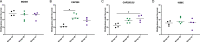



Similar articles
-
Antibody responses induced by SHIV infection are more focused than those induced by soluble native HIV-1 envelope trimers in non-human primates.PLoS Pathog. 2021 Aug 25;17(8):e1009736. doi: 10.1371/journal.ppat.1009736. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34432859 Free PMC article.
-
Immunogenicity of a Prefusion HIV-1 Envelope Trimer in Complex with a Quaternary-Structure-Specific Antibody.J Virol. 2015 Dec 30;90(6):2740-55. doi: 10.1128/JVI.02380-15. J Virol. 2015. PMID: 26719262 Free PMC article.
-
Closing and Opening Holes in the Glycan Shield of HIV-1 Envelope Glycoprotein SOSIP Trimers Can Redirect the Neutralizing Antibody Response to the Newly Unmasked Epitopes.J Virol. 2019 Feb 5;93(4):e01656-18. doi: 10.1128/JVI.01656-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487280 Free PMC article.
-
HIV-1 immunogens and strategies to drive antibody responses towards neutralization breadth.Retrovirology. 2018 Nov 26;15(1):74. doi: 10.1186/s12977-018-0457-7. Retrovirology. 2018. PMID: 30477581 Free PMC article. Review.
-
Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies.Retrovirology. 2018 Sep 12;15(1):63. doi: 10.1186/s12977-018-0445-y. Retrovirology. 2018. PMID: 30208933 Free PMC article. Review.
Cited by
-
Heterologous Immunization with Improved HIV-1 Subtype C Vaccines Elicit Autologous Tier 2 Neutralizing Antibodies with Rapid Viral Replication Control After SHIV Challenge.Viruses. 2025 Feb 17;17(2):277. doi: 10.3390/v17020277. Viruses. 2025. PMID: 40007032 Free PMC article.
-
Development of a Two-Component Nanoparticle Vaccine Displaying an HIV-1 Envelope Glycoprotein that Elicits Tier 2 Neutralising Antibodies.Vaccines (Basel). 2024 Sep 18;12(9):1063. doi: 10.3390/vaccines12091063. Vaccines (Basel). 2024. PMID: 39340093 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

