Interactive biocatalysis achieved by driving enzyme cascades inside a porous conducting material
- PMID: 38858478
- PMCID: PMC11165005
- DOI: 10.1038/s42004-024-01211-5
Interactive biocatalysis achieved by driving enzyme cascades inside a porous conducting material
Abstract
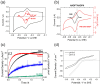
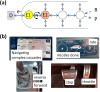
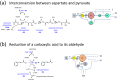
An emerging concept and platform, the electrochemical Leaf (e-Leaf), offers a radical change in the way tandem (multi-step) catalysis by enzyme cascades is studied and exploited. The various enzymes are loaded into an electronically conducting porous material composed of metallic oxide nanoparticles, where they achieve high concentration and crowding - in the latter respect the environment resembles that found in living cells. By exploiting efficient electron tunneling between the nanoparticles and one of the enzymes, the e-Leaf enables the user to interact directly with complex networks, rendering simultaneous the abilities to energise, control and observe catalysis. Because dispersion of intermediates is physically suppressed, the output of the cascade - the rate of flow of chemical steps and information - is delivered in real time as electrical current. Myriad enzymes of all major classes now become effectively electroactive in a technology that offers scalability between micro-(analytical, multiplex) and macro-(synthesis) levels. This Perspective describes how the e-Leaf was discovered, the steps in its development so far, and the outlook for future research and applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ricca E, Brucher B, Schrittwieser JH. Multi-enzymatic cascade reactions: overview and perspectives. Adv. Synth. Catal. 2011;353:2239–2262. doi: 10.1002/adsc.201100256. - DOI
-
- Lewis RD, France SP, Martinez CA. Emerging technologies for biocatalysis in the pharmaceutical industry. ACS Catal. 2023;13:5571–5577. doi: 10.1021/acscatal.3c00812. - DOI

