Tumour microenvironment programming by an RNA-RNA-binding protein complex creates a druggable vulnerability in IDH-wild-type glioblastoma
- PMID: 38858501
- PMCID: PMC11178504
- DOI: 10.1038/s41556-024-01428-5
Tumour microenvironment programming by an RNA-RNA-binding protein complex creates a druggable vulnerability in IDH-wild-type glioblastoma
Retraction in
-
Retraction Note: Tumour microenvironment programming by an RNA-RNA-binding protein complex creates a druggable vulnerability in IDH-wild-type glioblastoma.Nat Cell Biol. 2025 Mar;27(3):546. doi: 10.1038/s41556-025-01613-0. Nat Cell Biol. 2025. PMID: 39820034 Free PMC article. No abstract available.
Abstract
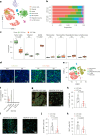
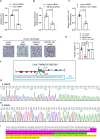
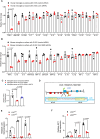
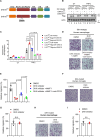
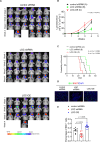
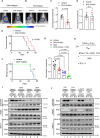
Patients with IDH-wild-type glioblastomas have a poor five-year survival rate along with limited treatment efficacy due to immune cell (glioma-associated microglia and macrophages) infiltration promoting tumour growth and resistance. To enhance therapeutic options, our study investigated the unique RNA-RNA-binding protein complex LOC-DHX15. This complex plays a crucial role in driving immune cell infiltration and tumour growth by establishing a feedback loop between cancer and immune cells, intensifying cancer aggressiveness. Targeting this complex with blood-brain barrier-permeable small molecules improved treatment efficacy, disrupting cell communication and impeding cancer cell survival and stem-like properties. Focusing on RNA-RNA-binding protein interactions emerges as a promising approach not only for glioblastomas without the IDH mutation but also for potential applications beyond cancer, offering new avenues for developing therapies that address intricate cellular relationships in the body.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

