Adverse effects of methylphenidate for apathy in patients with Alzheimer's disease (ADMET2 trial)
- PMID: 38858522
- PMCID: PMC11265565
- DOI: 10.1002/gps.6108
Adverse effects of methylphenidate for apathy in patients with Alzheimer's disease (ADMET2 trial)
Abstract
Objectives: To examine clinically important adverse events (AEs) associated with methylphenidate (MPH) treatment of apathy in Alzheimer's Disease (AD) versus placebo, including weight loss, vital signs, falls, and insomnia.
Methods: The Apathy in Dementia Methylphenidate Trial 2 (ADMET2) trial was a multicenter randomized, placebo-controlled trial of MPH to treat apathy in individuals with apathy and AD. Participants in ADMET2 had vital signs and weight measured at monthly visits through 6 months. AEs, including insomnia, falls, and cardiovascular events, were reported at every visit by participants and families using a symptom checklist.
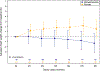
Results: The study included 98 participants in the MPH group and 101 in the placebo group. Participants in the MPH group experienced greater weight loss on average than the placebo through the 6-month follow-up, with a difference in change between MPH and placebo of 2.8 lb (95% confidence interval, CI: 0.7, 4.9 lb). No treatment group differences in change during the trial were found in systolic and diastolic blood pressure. More participants in the MPH group reported falls during the follow-up, 10 versus 6 in MPH and placebo groups, respectively. No differences in post-baseline insomnia were observed between the treatment groups. No participants reported instances of myocardial infarction, congestive heart failure, arrhythmia, stroke, or cardiomyopathy throughout the study period.
Conclusions: MPH use in AD patients for treating apathy is relatively safe, particularly notable given the many medical comorbidities in this population. There was a statistically significant but modest weight loss associated with MPH use, and clinicians are thus advised to monitor weight during MPH treatment.
Keywords: Alzheimer's disease; adverse events; apathy; methylphenidate; weight loss.
© 2024 John Wiley & Sons Ltd.
Figures
References
-
- Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry 2003;11(2):214–21. (In eng). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

