Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial
- PMID: 38858523
- PMCID: PMC11271400
- DOI: 10.1038/s41591-024-03018-2
Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial
Abstract
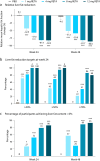
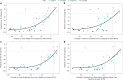
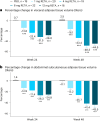
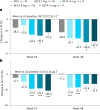
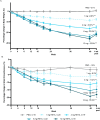
Retatrutide is a novel triple agonist of the glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1 and glucagon receptors. A 48-week phase 2 obesity study demonstrated weight reductions of 22.8% and 24.2% with retatrutide 8 and 12 mg, respectively. The primary objective of this substudy was to assess mean relative change from baseline in liver fat (LF) at 24 weeks in participants from that study with metabolic dysfunction-associated steatotic liver disease and ≥10% of LF. Here, in this randomized, double-blind, placebo-controlled trial, participants (n = 98) were randomly assigned to 48 weeks of once-weekly subcutaneous retatrutide (1, 4, 8 or 12 mg dose) or placebo. The mean relative change from baseline in LF at 24 weeks was -42.9% (1 mg), -57.0% (4 mg), -81.4% (8 mg), -82.4% (12 mg) and +0.3% (placebo) (all P < 0.001 versus placebo). At 24 weeks, normal LF (<5%) was achieved by 27% (1 mg), 52% (4 mg), 79% (8 mg), 86% (12 mg) and 0% (placebo) of participants. LF reductions were significantly related to changes in body weight, abdominal fat and metabolic measures associated with improved insulin sensitivity and lipid metabolism. The ClinicalTrials.gov registration is NCT04881760 .
© 2024. The Author(s).
Conflict of interest statement
A.J.S. has stock options in Genfit, Tiziana, Indalo, Durect, Inversago and Galmed. He has served as a consultant to AstraZeneca, Salix, Tobira, Takeda, Janssen, Gilead, Terns, Merck, Madrigal, NGM Biopharmaceuticals, Sagimet, Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Hemoshear, Novartis, Inventiva, Enyo, Akero, 89Bio, Novo Nordisk, Pfizer, Amgen, Genentech, Regeneron, Alnylam, Hanmi, LG Chem, Histoindex, Thera Technologies, Intercept, Target-RWE, Surrozen, Zydus, Path AI, Exhalenz and Genfit. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers Squibb, Pfizer, Intercept, Merck, AstraZeneca, Malinckrodt and Novartis. He receives royalties from Elsevier and UptoDate. L.M.K. serves as a scientific and medical consultant to Altimmune, Boehringer Ingelheim, Cytoki, Flagship Pioneering, Gilead, Glyscend, Intellihealth, Johnson & Johnson, Kallyope, Eli Lilly and Company, Novo Nordisk, Pfizer, Sidekick Health and twenty30.health. J.P.F. reports research funding from Akero, AstraZeneca, Boehringer Ingelheim, 89bio, Eli Lilly and Company, Intercept, Ionis, Janssen, Madrigal, Metacrine, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Poxel and Sanofi; consulting fees from Akero, Altimmune, Boehringer Ingelheim, Carmot Therapeutics, Echosens, 89bio, Eli Lilly and Company, Merck, Novo Nordisk, Pfizer and Sanofi; speaker bureau from Eli Lilly and Company; support for attending meetings or travel from Eli Lilly and Company, Novo Nordisk, Pfizer and Sanofi; participant advisory boards and consulting for Altimmune, Becton Dickinson, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly and Company, Gilead, Intercept, Merck, Novo Nordisk, Pfizer and Sanofi; and is on the board of directors for T1D Exchange. M.L.H., B.B., C.H., K.J.M., A.H., Q.W., Y.D. and N.C.S. are employees and shareholders of Eli Lilly and Company. M.K.T. is an employee and shareholder of Eli Lilly and Company and a steering committee member for Accelerating Medicines Partnership—Common Metabolic Diseases.
Figures






References
-
- Rinella, M. E. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol.10.1016/j.aohep.2023.101133 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

