Estrogen counteracts age-related decline in beige adipogenesis through the NAMPT-regulated ER stress response
- PMID: 38858606
- PMCID: PMC11829733
- DOI: 10.1038/s43587-024-00633-z
Estrogen counteracts age-related decline in beige adipogenesis through the NAMPT-regulated ER stress response
Abstract
Thermogenic beige adipocytes are recognized as potential therapeutic targets for combating metabolic diseases. However, the metabolic advantages that they offer are compromised with aging. Here we show that treating mice with estrogen (E2), a hormone that decreases with age, can counteract the age-related decline in beige adipogenesis when exposed to cold temperature while concurrently enhancing energy expenditure and improving glucose tolerance in mice. Mechanistically, we found that nicotinamide phosphoribosyl transferase (NAMPT) plays a pivotal role in facilitating the formation of E2-induced beige adipocytes, which subsequently suppresses the onset of age-related endoplasmic reticulum (ER) stress. Furthermore, we found that targeting NAMPT signaling, either genetically or pharmacologically, can restore the formation of beige adipocytes by increasing the number of perivascular adipocyte progenitor cells. Conversely, the absence of NAMPT signaling prevents this process. Together, our findings shed light on the mechanisms regulating the age-dependent impairment of beige adipocyte formation and underscore the E2-NAMPT-controlled ER stress pathway as a key regulator of this process.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no conflict of interest.
Figures

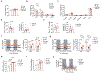
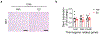




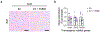










References
-
- Spiegelman BM & Flier JS Obesity and the regulation of energy balance. Cell 104, 531–543 (2001). https://doi.org:S0092-8674(01)00240-9 [pii] - PubMed
-
- Friedman JM Obesity: Causes and control of excess body fat. Nature 459, 340–342 (2009). https://doi.org.459340a [pii] 10.1038/459340a - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK123098/DK/NIDDK NIH HHS/United States
- R01 HL148756/HL/NHLBI NIH HHS/United States
- P30DK020595/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01HL148756/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01DK123098/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- P30 DK020595/DK/NIDDK NIH HHS/United States
- R01DK132398/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 DK132398/DK/NIDDK NIH HHS/United States
- K01 DK111771/DK/NIDDK NIH HHS/United States
- K01 OD011177/OD/NIH HHS/United States
- R03DK127149/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R03 DK127149/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

