Uptake, effectiveness and safety of COVID-19 vaccines in individuals at clinical risk due to immunosuppressive drug therapy or transplantation procedures: a population-based cohort study in England
- PMID: 38858672
- PMCID: PMC11165729
- DOI: 10.1186/s12916-024-03457-1
Uptake, effectiveness and safety of COVID-19 vaccines in individuals at clinical risk due to immunosuppressive drug therapy or transplantation procedures: a population-based cohort study in England
Abstract
Background: Immunocompromised individuals are at increased risk of severe COVID-19 outcomes, underscoring the importance of COVID-19 vaccination in this population. The lack of comprehensive real-world data on vaccine uptake, effectiveness and safety in these individuals presents a critical knowledge gap, highlighting the urgency to better understand and address the unique challenges faced by immunocompromised individuals in the context of COVID-19 vaccination.
Methods: We analysed data from 12,274,946 people in the UK aged > 12 years from 01/12/2020 to 11/04/2022. Of these, 583,541 (4.8%) were immunocompromised due to immunosuppressive drugs, organ transplants, dialysis or chemotherapy. We undertook a cohort analysis to determine COVID-19 vaccine uptake, nested case-control analyses adjusted for comorbidities and sociodemographic characteristics to determine effectiveness of vaccination against COVID-19 hospitalisation, ICU admission and death, and a self-controlled case series assessing vaccine safety for pre-specified adverse events of interest.
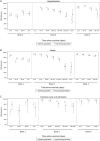
Results: Overall, 93.7% of immunocompromised individuals received at least one COVID-19 vaccine dose, with 80.4% having received three or more doses. Uptake reduced with increasing deprivation (hazard ratio [HR] 0.78 [95%CI 0.77-0.79] in the most deprived quintile compared to the least deprived quintile for the first dose). Estimated vaccine effectiveness against COVID-19 hospitalisation 2-6 weeks after the second and third doses compared to unvaccinated was 78% (95%CI 72-83) and 91% (95%CI 88-93) in the immunocompromised population, versus 85% (95%CI 83-86) and 86% (95%CI 85-89), respectively, for the general population. Results showed COVID-19 vaccines were protective against intensive care unit (ICU) admission and death in both populations, with effectiveness of over 92% against COVID-19-related death and up to 95% in reducing ICU admissions for both populations following the third dose. COVID-19 vaccines were generally safe for immunocompromised individuals, though specific doses of ChAdOx1, mRNA-1273 and BNT162b2 raised risks of specific cardiovascular/neurological conditions.
Conclusions: COVID-19 vaccine uptake is high in immunocompromised individuals on immunosuppressive drug therapy or who have undergone transplantation procedures, with documented disparities by deprivation. Findings suggest that COVID-19 vaccines are protective against severe COVID-19 outcomes in this vulnerable population, and show a similar safety profile in immunocompromised individuals and the general population, despite some increased risk of adverse events. These results underscore the importance of ongoing vaccination prioritisation for this clinically at-risk population to maximise protection against severe COVID-19 outcomes.
Keywords: COVID-19; COVID-19 vaccination; Immunocompromised; Population-based; Vaccine effectiveness; Vaccine safety; Vaccine uptake.
© 2024. The Author(s).
Conflict of interest statement
JHC reports grants from National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford, grants from John Fell Oxford University Press Research Fund, and other research councils, during the conduct of the study. JHC is an unpaid director of QResearch, a not-for-profit organisation which is a partnership between the University of Oxford and EMIS Health who supply the QResearch database used for this work. Until 09 Aug 2023, JHC had a 50% shareholding in ClinRisk Ltd, co-owning it with her husband, who was an executive director. On 9th August 2023, 100% of the share capital was donated to Endeavour Health Care Charitable Trust and the company renamed to Endeavour Predict Ltd. JHC is an unpaid consultant to Endeavour Predict Ltd and her husband is a non-executive director to cover the transition. Endeavour Predict Ltd produces open and closed source software to implement clinical risk algorithms (outside this work) into clinical computer systems. All other authors declare no competing interests.
Figures



References
-
- UK Health Security Agency. COVID-19 vaccine surveillance report week 17. 28 April 2022. 2022. Available from: https://assets.publishing.service.gov.uk/media/626a7dc08fa8f57a33ccecb6/....
-
- Eder L, Croxford R, Drucker AM, Mendel A, Kuriya B, Touma Z, et al. COVID-19 hospitalizations, intensive care unit stays, ventilation, and death among patients with immune-mediated inflammatory diseases compared to controls. J Rheumatol. 2022;49(5):523–530. doi: 10.3899/jrheum.211012. - DOI - PubMed
-
- Fagni F, Simon D, Tascilar K, Schoenau V, Sticherling M, Neurath MF, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3(10):e724–e736. doi: 10.1016/S2665-9913(21)00247-2. - DOI - PMC - PubMed
-
- Nab L, Parker EPK, Andrews CD, Hulme WJ, Fisher L, Morley J, et al. Changes in COVID-19-related mortality across key demographic and clinical subgroups in England from 2020 to 2022: a retrospective cohort study using the OpenSAFELY platform. Lancet Public Health. 2023;8(5):e364–e377. doi: 10.1016/S2468-2667(23)00079-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

