Response surface methodology mediated optimization of phytosulfokine and plant growth regulators for enhanced protoplast division, callus induction, and somatic embryogenesis in Angelica Gigas Nakai
- PMID: 38858674
- PMCID: PMC11165744
- DOI: 10.1186/s12870-024-05243-w
Response surface methodology mediated optimization of phytosulfokine and plant growth regulators for enhanced protoplast division, callus induction, and somatic embryogenesis in Angelica Gigas Nakai
Abstract
Background: Angelica Gigas (Purple parsnip) is an important medicinal plant that is cultivated and utilized in Korea, Japan, and China. It contains bioactive substances especially coumarins with anti-inflammatory, anti-platelet aggregation, anti-cancer, anti-diabetic, antimicrobial, anti-obesity, anti-oxidant, immunomodulatory, and neuroprotective properties. This medicinal crop can be genetically improved, and the metabolites can be obtained by embryonic stem cells. In this context, we established the protoplast-to-plant regeneration methodology in Angelica gigas.
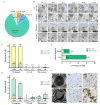
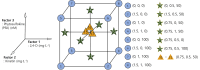
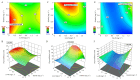
Results: In the present investigation, we isolated the protoplast from the embryogenic callus by applying methods that we have developed earlier and established protoplast cultures using Murashige and Skoog (MS) liquid medium and by embedding the protoplast in thin alginate layer (TAL) methods. We supplemented the culture medium with growth regulators namely 2,4-dichlorophenoxyaceticacid (2,4-D, 0, 0.75, 1.5 mg L- 1), kinetin (KN, 0, 0.5, and 1.0 mg L- 1) and phytosulfokine (PSK, 0, 50, 100 nM) to induce protoplast division, microcolony formation, and embryogenic callus regeneration. We applied central composite design (CCD) and response surface methodology (RSM) for the optimization of 2,4-D, KN, and PSK levels during protoplast division, micro-callus formation, and induction of embryogenic callus stages. The results revealed that 0.04 mg L- 1 2,4-D + 0.5 mg L- 1 KN + 2 nM PSK, 0.5 mg L- 1 2,4-D + 0.9 mg L- 1 KN and 90 nM PSK, and 1.5 mg L- 1 2,4-D and 1 mg L- 1 KN were optimum for protoplast division, micro-callus formation and induction embryogenic callus. MS basal semi-solid medium without growth regulators was good for the development of embryos and plant regeneration.
Conclusions: This study demonstrated successful protoplast culture, protoplast division, micro-callus formation, induction embryogenic callus, somatic embryogenesis, and plant regeneration in A. gigas. The methodologies developed here are quite useful for the genetic improvement of this important medicinal plant.
Keywords: Angelica gigas; Phytosulfokine; Protoplast culture; Response surface methodology; Somatic embryogenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Promotive effect of phytosulfokine - peptide growth factor - on protoplast cultures development in Fagopyrum tataricum (L.) Gaertn.BMC Plant Biol. 2023 Aug 10;23(1):385. doi: 10.1186/s12870-023-04402-9. BMC Plant Biol. 2023. PMID: 37563739 Free PMC article.
-
Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima Chi.J Plant Physiol. 2013 Sep 1;170(13):1202-11. doi: 10.1016/j.jplph.2013.03.019. Epub 2013 Jun 20. J Plant Physiol. 2013. PMID: 23790533
-
Composition of the Reconstituted Cell Wall in Protoplast-Derived Cells of Daucus is Affected by Phytosulfokine (PSK).Int J Mol Sci. 2019 Nov 4;20(21):5490. doi: 10.3390/ijms20215490. Int J Mol Sci. 2019. PMID: 31690047 Free PMC article.
-
Somatic Embryogenesis in the Family Gentianaceae and Its Biotechnological Application.Front Plant Sci. 2019 Jun 11;10:762. doi: 10.3389/fpls.2019.00762. eCollection 2019. Front Plant Sci. 2019. PMID: 31244878 Free PMC article. Review.
-
History and current status of embryogenic culture-based tissue culture, transformation and gene editing of maize (Zea mays L.).Plant Genome. 2025 Mar;18(1):e20451. doi: 10.1002/tpg2.20451. Epub 2024 Apr 11. Plant Genome. 2025. PMID: 38600860 Free PMC article. Review.
Cited by
-
Establishment of Efficient Somatic Embryo Maturation System of Pinus elliottii.Plants (Basel). 2025 Jun 29;14(13):1985. doi: 10.3390/plants14131985. Plants (Basel). 2025. PMID: 40647994 Free PMC article.
References
-
- Wang J, Jiang J, Wang Y. Protoplast fusion for crop improvement and breeding in China. Plant Cell Tissue Organ Cult. 2013;112:131–42. doi: 10.1007/s11240-012-0221-y. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

