The walnut-derived peptide TW-7 improves mouse parthenogenetic embryo development of vitrified MII oocytes potentially by promoting histone lactylation
- PMID: 38858724
- PMCID: PMC11165821
- DOI: 10.1186/s40104-024-01045-0
The walnut-derived peptide TW-7 improves mouse parthenogenetic embryo development of vitrified MII oocytes potentially by promoting histone lactylation
Abstract
Background: Previous studies have shown that the vitrification of metaphase II (MII) oocytes significantly represses their developmental potential. Abnormally increased oxidative stress is the probable factor; however, the underlying mechanism remains unclear. The walnut-derived peptide TW-7 was initially isolated and purified from walnut protein hydrolysate. Accumulating evidences implied that TW-7 was a powerful antioxidant, while its prospective application in oocyte cryopreservation has not been reported.
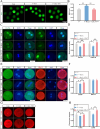
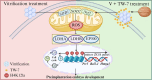
Result: Here, we found that parthenogenetic activation (PA) zygotes derived from vitrified MII oocytes showed elevated ROS level and delayed progression of pronucleus formation. Addition of 25 μmol/L TW-7 in warming, recovery, PA, and embryo culture medium could alleviate oxidative stress in PA zygotes from vitrified mouse MII oocytes, furtherly increase proteins related to histone lactylation such as LDHA, LDHB, and EP300 and finally improve histone lactylation in PA zygotes. The elevated histone lactylation facilitated the expression of minor zygotic genome activation (ZGA) genes and preimplantation embryo development.
Conclusions: Our findings revealed the mechanism of oxidative stress inducing repressed development of PA embryos from vitrified mouse MII oocytes and found a potent and easy-obtained short peptide that could significantly rescue the decreased developmental potential of vitrified oocytes, which would potentially contribute to reproductive medicine, animal protection, and breeding.
Keywords: Histone lactylation; Oocyte; TW-7; Vitrification; Zygotic genome activation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
TW-7 Alleviate Apoptosis of Embryos Derived From Vitrified MII Oocytes by Downregulation of JNK Signaling in Mice.FASEB J. 2025 Jun 15;39(11):e70712. doi: 10.1096/fj.202500222R. FASEB J. 2025. PMID: 40497346
-
Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification.Mol Hum Reprod. 2016 Dec;22(12):867-881. doi: 10.1093/molehr/gaw059. Epub 2016 Sep 7. Mol Hum Reprod. 2016. PMID: 27604460
-
Melatonin improves the first cleavage of parthenogenetic embryos from vitrified-warmed mouse oocytes potentially by promoting cell cycle progression.J Anim Sci Biotechnol. 2021 Jul 16;12(1):84. doi: 10.1186/s40104-021-00605-y. J Anim Sci Biotechnol. 2021. PMID: 34266479 Free PMC article.
-
Reduced competence of immature and mature oocytes vitrified by Cryotop method: assessment by in vitro fertilization and parthenogenetic activation in a bovine model.Zygote. 2017 Apr;25(2):222-230. doi: 10.1017/S0967199416000381. Epub 2017 Jan 10. Zygote. 2017. PMID: 28069092
-
Improved development by melatonin treatment after vitrification of mouse metaphase II oocytes.Cryobiology. 2016 Dec;73(3):335-342. doi: 10.1016/j.cryobiol.2016.09.171. Epub 2016 Oct 8. Cryobiology. 2016. PMID: 27725165
Cited by
-
Post-Translational Modifications in Mammalian Folliculogenesis and Ovarian Pathologies.Cells. 2025 Aug 20;14(16):1292. doi: 10.3390/cells14161292. Cells. 2025. PMID: 40862771 Free PMC article. Review.
-
Histone lactylation promotes multidrug resistance in hepatocellular carcinoma by forming a positive feedback loop with PTEN.Cell Death Dis. 2025 Jan 31;16(1):59. doi: 10.1038/s41419-025-07359-9. Cell Death Dis. 2025. PMID: 39890782 Free PMC article.
-
A comprehensive review of histone modifications during mammalian oogenesis and early embryo development.Histochem Cell Biol. 2025 Jun 28;163(1):70. doi: 10.1007/s00418-025-02398-x. Histochem Cell Biol. 2025. PMID: 40580240 Free PMC article. Review.
-
Lactylation: From Molecular Insights to Disease Relevance.Biomolecules. 2025 Jun 3;15(6):810. doi: 10.3390/biom15060810. Biomolecules. 2025. PMID: 40563450 Free PMC article. Review.
References
-
- Borrás A, Manau D, Fabregues F, Peralta S, Calafell JM, Casals G, et al. Comparison between slow freezing and vitrification of ovarian tissue cryopreservation in assigned female at birth transgender people receiving testosterone therapy: data on histological and viability parameters. J Assist Reprod Genet. 2022;39(2):527–541. doi: 10.1007/s10815-021-02386-9. - DOI - PMC - PubMed
-
- Barrozo LG, Paulino L, Silva BR, Barbalho EC, Nascimento DR, Neto MFL, et al. N-acetyl-cysteine and the control of oxidative stress during in vitro ovarian follicle growth, oocyte maturation, embryo development and cryopreservation. Anim Reprod Sci. 2021;231:106801. doi: 10.1016/j.anireprosci.2021.106801. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

