The IL-33-ST2 axis plays a vital role in endometriosis via promoting epithelial-mesenchymal transition by phosphorylating β-catenin
- PMID: 38858740
- PMCID: PMC11163813
- DOI: 10.1186/s12964-024-01683-x
The IL-33-ST2 axis plays a vital role in endometriosis via promoting epithelial-mesenchymal transition by phosphorylating β-catenin
Abstract
Objectives: Interleukin 33 (IL-33) is a crucial inflammatory factor that functions as an alarm signal in endometriosis (EMs). Epithelial-mesenchymal transition (EMT), a process related to inflammatory signals, intracellular reactive oxygen species (ROS) production, and lipid peroxidation, have been proposed as potential mechanisms that contribute to the development and progression of EMs. IL-33 is highly upregulated in the ectopic milieu. Moreover, ectopic endometrial cells constitutively express interleukin-33 receptor ST2 (IL-33R). However, the role of IL-33/ST2 in the EMT of EMs remains largely unknown. In this study, we aimed to mechanistically determine the role of IL-33/ST2 in EMs-associated fibrosis.
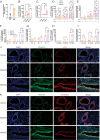
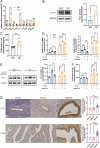
Materials and methods: We established a non-lethal oxidative stress model to explore the conditions that trigger IL-33 induction. We performed α-smooth muscle actin (α-SMA) protein detection, cell counting kit-8 (CCK-8) assays, and scratch assays to analyze the impact of IL-33 on primary endometrial stromal cells (ESCs) proliferation and invasion. Clinical samples from patients with or without EMs were subjected to immunohistochemical (IHC) and and immunofluorescence(IF) staining to assess the clinical relevance of IL-33 receptor ST2 and EMT-related proteins. Furthermore, we used the ectopic human endometrial epithelial cell line 12Z and normal human epithelial cell line EEC to evaluate the effects of IL-33 on Wnt/β-catenin signaling. The effect of IL-33 on EMT-associated fibrosis was validated in vivo by intraperitoneal injections of IL-33 and antiST2.
Results: We observed that ectopic milieu, characterized by ROS, TGF-β1, and high level of estrogen, triggers the secretion of IL-33 from ectopic ESCs. Ectopic endometrial lesions exhibited higher level of fibrotic characteristics and ST2 expression than that in the normal endometrium. Exogenous recombinant human (rhIL-33) enhanced ESC migration and survival. Similarly, 12Z cells displayed a higher degree of EMT characteristics with elevated expression of CCN4 and Fra-1, downstream target genes of the WNT/β-catenin pathway, than that observed in EECs. Conversely, blocking IL-33 with neutralizing antibodies, knocking down ST2 or β-catenin with siRNA, and β-catenin dephosphorylation abolished its effects on EMT promotion. In vivo validation demonstrated that IL-33 significantly promotes EMs-related fibrosis through the activation of Wnt/β-catenin signaling.
Conclusion: Our data strongly support the vital role of the IL-33/ST2 pathway in EMs-associated fibrosis and emphasize the importance of the EMT in the pathophysiology of fibrosis. Targeting the IL-33/ST2/Wnt/β-catenin axis may hold promise as a feasible therapeutic approach for controlling fibrosis in EMs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Doroftei B, Ilie OD, Balmus IM, Ciobica A, Maftei R, Scripcariu I, Simionescu G, Grab D, Stoian I, Ilea C. Molecular and clinical insights on the complex interaction between oxidative stress, apoptosis, and Endobiota in the pathogenesis of endometriosis. Diagnostics (Basel) 2021;11(8):1434. doi: 10.3390/diagnostics11081434. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

