Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomized trial
- PMID: 38858818
- PMCID: PMC11852274
- DOI: 10.1093/ndt/gfae132
Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomized trial
Abstract
Background: Finerenone, a non-steroidal mineralocorticoid receptor antagonist, improved kidney and cardiovascular outcomes in patients with chronic kidney disease (CKD) and type 2 diabetes in two phase 3 outcome trials. The Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) study investigates the effect of finerenone in adults with CKD without diabetes.
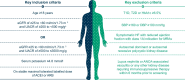
Methods: FIND-CKD (NCT05047263 and EU CT 2023-506897-11-00) is a randomized, double-blind, placebo-controlled phase 3 trial in patients with CKD of non-diabetic aetiology. Adults with a urinary albumin:creatinine ratio (UACR) ≥200-≤3500 mg/g and an estimated glomerular filtration rate (eGFR) ≥25-<90 ml/min/1.73 m2 receiving a maximum tolerated dose of a renin-angiotensin system inhibitor were randomized 1:1 to once-daily placebo or finerenone 10 or 20 mg depending on eGFR >60 or <60 ml/min/1.73 m2. The primary efficacy outcome is total eGFR slope, defined as the mean annual rate of change in eGFR from baseline to month 32. Secondary efficacy outcomes include a combined cardiorenal composite outcome comprising time to kidney failure, sustained ≥57% decrease in eGFR, hospitalization for heart failure or cardiovascular death, as well as separate kidney and cardiovascular composite outcomes. Adverse events are recorded to assess tolerability and safety.
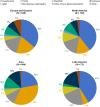
Results: Across 24 countries, 3231 patients were screened and 1584 were randomized to study treatment. The most common causes of CKD were chronic glomerulonephritis (57.0%) and hypertensive/ischaemic nephropathy (29.0%). Immunoglobulin A nephropathy was the most common glomerulonephritis (26.3% of the total population). At baseline, mean eGFR and median UACR were 46.7 ml/min/1.73 m2 and 818.9 mg/g, respectively. Diuretics were used by 282 participants (17.8%), statins by 851 (53.7%) and calcium channel blockers by 794 (50.1%). Sodium-glucose co-transporter 2 (SGLT2) inhibitors were used in 16.9% of patients; these individuals had a similar mean eGFR (45.6 versus 46.8 ml/min/1.73 m2) and a slightly higher median UACR (871.9 versus 808.3 mg/g) compared with those not using SGLT2 inhibitors at baseline.
Conclusions: FIND-CKD is the first phase 3 trial of finerenone in patients with CKD of non-diabetic aetiology.
Keywords: clinical trial; eGFR slope; finerenone; immunoglobulin A nephropathy; non-diabetic chronic kidney disease.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
This study is sponsored by Bayer AG. The authors wrote the article with the assistance of a medical writer funded by the sponsor. The sponsor was involved in the study design and the writing of the report. H.J.L.H. has received grants from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk; consulting fees from AstraZeneca, Alexion, Bayer, Boehringer Ingelheim, CSL Behring, Chinook, Dimerix, Eli Lilly, Gilead, Janssen, Merck, Mitsubishi Tanabe, Novartis, Novo Nordisk and Travere Therapeutics and payments or honoraria for speaking from AstraZeneca. R.A. has received personal fees and non-financial support from Bayer Healthcare Pharmaceuticals; personal fees and non-financial support from Akebia Therapeutics, Boehringer Ingelheim, Eli Lilly and Vifor Pharma; serves as a member of data safety monitoring committees for Chinook and Vertex Pharmaceuticals; serves as a member of steering committees of randomized trials for Akebia Therapeutics and Bayer; serves as Associate Editor for the
Figures


References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. https://kdigo.org/wp-content/uploads/2024/03/KDIGO-2024-CKD-Guideline.pdf (June 2024, date last accessed). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

