Serum neurofilament light chain in distinct phenotypes of amyotrophic lateral sclerosis: A longitudinal, multicenter study
- PMID: 38859579
- PMCID: PMC11295170
- DOI: 10.1111/ene.16379
Serum neurofilament light chain in distinct phenotypes of amyotrophic lateral sclerosis: A longitudinal, multicenter study
Abstract
Objective: To assess the performance of serum neurofilament light chain (sNfL) in clinical phenotypes of amyotrophic lateral sclerosis (ALS).
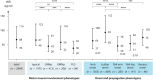
Methods: In 2949 ALS patients at 16 ALS centers in Germany and Austria, clinical characteristics and sNfL were assessed. Phenotypes were differentiated for two anatomical determinants: (1) upper and/or lower motor involvement (typical, typMN; upper/lower motor neuron predominant, UMNp/LMNp; primary lateral sclerosis, PLS) and (2) region of onset and propagation of motor neuron dysfunction (bulbar, limb, flail-arm, flail-leg, thoracic onset). Phenotypes were correlated to sNfL, progression, and survival.
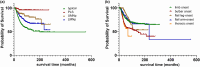
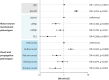
Results: Mean sNfL was - compared to typMN (75.7 pg/mL, n = 1791) - significantly lower in LMNp (45.1 pg/mL, n = 413), UMNp (58.7 pg/mL n = 206), and PLS (37.6 pg/mL, n = 84). Also, sNfL significantly differed in the bulbar (92.7 pg/mL, n = 669), limb (64.1 pg/mL, n = 1305), flail-arm (46.4 pg/mL, n = 283), flail-leg (53.6 pg/mL, n = 141), and thoracic (74.5 pg/mL, n = 96) phenotypes. Binary logistic regression analysis showed highest contribution to sNfL elevation for faster progression (odds ratio [OR] 3.24) and for the bulbar onset phenotype (OR 1.94). In contrast, PLS (OR 0.20), LMNp (OR 0.45), and thoracic onset (OR 0.43) showed reduced contributions to sNfL. Longitudinal sNfL (median 12 months, n = 2862) showed minor monthly changes (<0.2%) across all phenotypes. Correlation of sNfL with survival was confirmed (p < 0.001).
Conclusions: This study underscored the correlation of ALS phenotypes - differentiated for motor neuron involvement and region of onset/propagation - with sNfL, progression, and survival. These phenotypes demonstrated a significant effect on sNfL and should be recognized as independent confounders of sNfL analyses in ALS trials and clinical practice.
Keywords: NfL; amyotrophic lateral sclerosis; biomarker; phenotype; serum neurofilament light chain.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
T.M. has received grants, personal fees, non‐financial support, and research support from AL‐S Pharma, Amylyx, Cytokinetics, Ferrer, Mitsubishi Tanabe, Sanofi, Orphazyme, and served on the advisory boards of Amylyx, Biogen, and ITF Pharma outside of the submitted work. T.M. and C.M. are founders and shareholders of the Ambulanzpartner Soziotechnologie APST GmbH, which makes the mobile application “ALS‐App.” T.G. has received personal fees from ITF Pharma and served on the advisory boards of Amylyx and ITF Pharma outside of the submitted work. P.W. has served on advisory boards of Biogen, ITF Pharma, and Novartis outside of the submitted work. R.G. has received grants, personal fees, non‐financial support, and research support from Biogen and served on the advisory boards of Biogen, Roche, and ITF Pharma outside of the submitted work. P.L. has received consulting fees from AbbVie, Alexion, BIAL, Desitin, ITF Pharma, STADA Pharm, Woolsey Pharmaceuticals, and Zambon outside of the submitted work. He is co‐inventor on patents EP 2825175 B1, US 9.980,972 B2 for the use of Fasudil in ALS. J.C.K. has received consulting fees and compensations for talks from Biogen, Roche, and AbbVie and has served on advisory boards for Biogen and Roche. S.P. has received speaker fees, non‐financial support, and research support from Biogen, Roche, ALS Pharma, Amylyx, Cytokinetics, Ferrer, ITF Pharma, and Sanofi and served on advisory boards of Amylyx, Biogen, Roche, Zambon, and ITF Pharma outside of the submitted work. A.H. has received funding from the European Social Fonds, the Federal Ministry of Education and Research, and the “Hermann und Lilly Schilling‐Stiftung für medizinische Forschung im Stifterverband”, honoraria for presentations/advisory boards from Amylyx, Desitin, and ITF Pharma, and royalties from Elsevier Press and Kohlhammer. J.P. has received consulting fees from Hormosan Pharma. M.B. has served on advisory boards for Sanofi, Amicus, and Biogen and has received speaker honoraria from Sanofi, Amicus, ITF Pharma, and Biogen, and financial research support from Sanofi and Löwenstein Medical, all outside of the submitted work. J.D. has received speaker honoraria from Biogen, ITF Pharma, and Zambon. I.C. received personal fees from Biogen and Roche (speaker honoraria and/or participation in advisory boards) outside the submitted work. A.L. has served on advisory boards of Roche, Biogen, Alector, and Amylyx and received compensation from Biologix, German Society of Neurology, Biogen, Springer Medicine, Amylyx, and Streamed Up!, and financial research support from Amylyx, Biogen, Ferrer International, Novartis, Mitsubishi Tanabe, Apellis Pharmaceuticals, Alexion, Orion Pharma, Orphazyme, the European Union, and BMBF. P.K. received consulting fees from Biogen. R.S. has received honoraria for presentations from ITF Pharma outside of the submitted work. A.M. has received personal fees, non‐financial support, and research support from ITF Pharma and Zambon outside the submitted work. The other authors declare no conflicts of interest.
Figures


References
-
- van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084‐2098. - PubMed
-
- Chio A, Calvo A, Moglia C, Mazzini L, Mora G, PARALS Study Group . Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740‐746. - PubMed
-
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10:661‐670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

