Robust Quantification of Live-Cell Single-Molecule Tracking Data for Fluorophores with Different Photophysical Properties
- PMID: 38859654
- PMCID: PMC11301680
- DOI: 10.1021/acs.jpcb.4c01454
Robust Quantification of Live-Cell Single-Molecule Tracking Data for Fluorophores with Different Photophysical Properties
Abstract
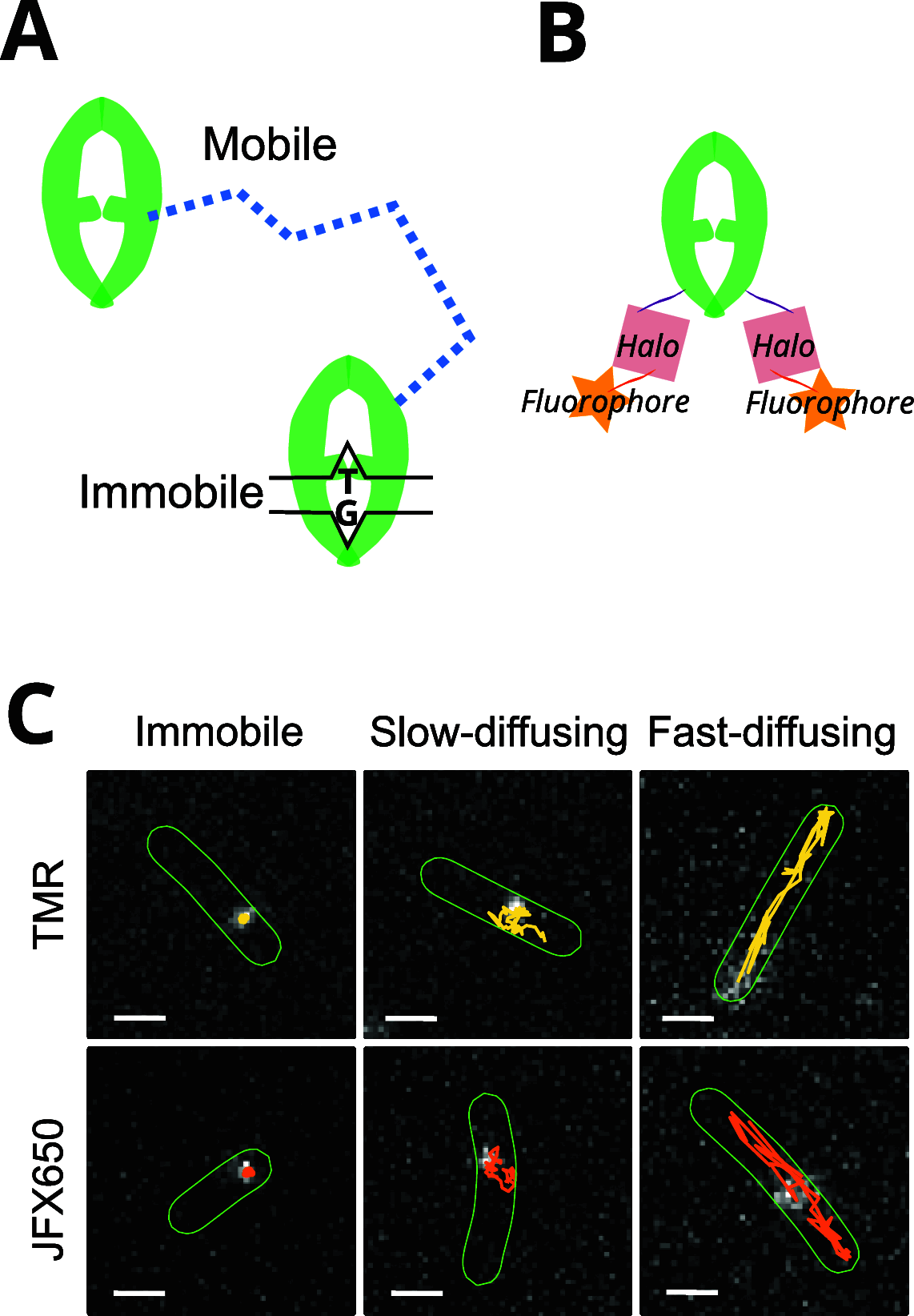
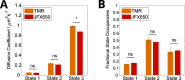
High-speed single-molecule tracking in live cells is becoming an increasingly popular method for quantifying the spatiotemporal behavior of proteins in vivo. The method provides a wealth of quantitative information, but users need to be aware of biases that can skew estimates of molecular mobilities. The range of suitable fluorophores for live-cell single-molecule imaging has grown substantially over the past few years, but it remains unclear to what extent differences in photophysical properties introduce biases. Here, we tested two fluorophores with entirely different photophysical properties, one that photoswitches frequently between bright and dark states (TMR) and one that shows exceptional photostability without photoswitching (JFX650). We used a fusion of the Escherichia coli DNA repair enzyme MutS to the HaloTag and optimized sample preparation and imaging conditions for both types of fluorophore. We then assessed the reliability of two common data analysis algorithms, mean-square displacement (MSD) analysis and Hidden Markov Modeling (HMM), to estimate the diffusion coefficients and fractions of MutS molecules in different states of motion. We introduce a simple approach that removes discrepancies in the data analyses and show that both algorithms yield consistent results, regardless of the fluorophore used. Nevertheless, each dye has its own strengths and weaknesses, with TMR being more suitable for sampling the diffusive behavior of many molecules, while JFX650 enables prolonged observation of only a few molecules per cell. These characterizations and recommendations should help to standardize measurements for increased reproducibility and comparability across studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Detecting molecular interactions in live-cell single-molecule imaging with proximity-assisted photoactivation (PAPA).Elife. 2022 Aug 17;11:e76870. doi: 10.7554/eLife.76870. Elife. 2022. PMID: 35976226 Free PMC article.
-
Evaluation of fluorophores to label SNAP-tag fused proteins for multicolor single-molecule tracking microscopy in live cells.Biophys J. 2014 Aug 19;107(4):803-14. doi: 10.1016/j.bpj.2014.06.040. Biophys J. 2014. PMID: 25140415 Free PMC article.
-
Cascading MutS and MutL sliding clamps control DNA diffusion to activate mismatch repair.Nature. 2016 Nov 24;539(7630):583-587. doi: 10.1038/nature20562. Epub 2016 Nov 16. Nature. 2016. PMID: 27851738 Free PMC article.
-
Photostable and photoswitching fluorescent dyes for super-resolution imaging.J Biol Inorg Chem. 2017 Jul;22(5):639-652. doi: 10.1007/s00775-016-1435-y. Epub 2017 Jan 12. J Biol Inorg Chem. 2017. PMID: 28083655 Review.
-
Optically modulated fluorescence bioimaging: visualizing obscured fluorophores in high background.Acc Chem Res. 2014 May 20;47(5):1545-54. doi: 10.1021/ar400325y. Epub 2014 Apr 14. Acc Chem Res. 2014. PMID: 24725021 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

