Cortical signatures of auditory looming bias show cue-specific adaptation between newborns and young adults
- PMID: 38859821
- PMCID: PMC11163589
- DOI: 10.1038/s44271-024-00105-5
Cortical signatures of auditory looming bias show cue-specific adaptation between newborns and young adults
Abstract
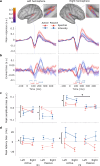
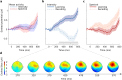
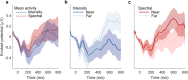
Adaptive biases in favor of approaching, or "looming", sounds have been found across ages and species, thereby implicating the potential of their evolutionary origin and universal basis. The human auditory system is well-developed at birth, yet spatial hearing abilities further develop with age. To disentangle the speculated inborn, evolutionary component of the auditory looming bias from its learned counterpart, we collected high-density electroencephalographic data across human adults and newborns. As distance-motion cues we manipulated either the sound's intensity or spectral shape, which is pinna-induced and thus prenatally inaccessible. Through cortical source localisation we demonstrated the emergence of the bias in both age groups at the level of Heschl's gyrus. Adults exhibited the bias in both attentive and inattentive states; yet differences in amplitude and latency appeared based on attention and cue type. Contrary to the adults, in newborns the bias was elicited only through manipulations of intensity and not spectral cues. We conclude that the looming bias comprises innate components while flexibly incorporating the spatial cues acquired through lifelong exposure.
Keywords: Cortex; Human behaviour.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures





Similar articles
-
Asymmetries in behavioral and neural responses to spectral cues demonstrate the generality of auditory looming bias.Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):9743-9748. doi: 10.1073/pnas.1703247114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827336 Free PMC article.
-
Frontal cortex selectively overrides auditory processing to bias perception for looming sonic motion.Brain Res. 2020 Jan 1;1726:146507. doi: 10.1016/j.brainres.2019.146507. Epub 2019 Oct 10. Brain Res. 2020. PMID: 31606413 Free PMC article.
-
Auditory motion in depth is preferentially 'captured' by visual looming signals.Seeing Perceiving. 2012;25(1):71-85. doi: 10.1163/187847611X620928. Seeing Perceiving. 2012. PMID: 22353569
-
Stimulus-dependent activations and attention-related modulations in the auditory cortex: a meta-analysis of fMRI studies.Hear Res. 2014 Jan;307:29-41. doi: 10.1016/j.heares.2013.08.001. Epub 2013 Aug 11. Hear Res. 2014. PMID: 23938208 Review.
-
Relationships between human auditory cortical structure and function.Audiol Neurootol. 2003 Jan-Feb;8(1):1-18. doi: 10.1159/000067894. Audiol Neurootol. 2003. PMID: 12566688 Review.
Cited by
-
Bayesian prior uncertainty and surprisal elicit distinct neural patterns during sound localization in dynamic environments.Sci Rep. 2025 Mar 7;15(1):7931. doi: 10.1038/s41598-025-90269-9. Sci Rep. 2025. PMID: 40050310 Free PMC article.
References
-
- Haselton, M. G., Nettle, D. & Murray, D. R. The Evolution of Cognitive Bias. The Handbook of Evolutionary Psychology 968–987 (2015).
-
- Neuhoff, J. G. Adaptive biases in visual and auditory looming perception. In (ed Hubbard, T. L.) Spatial Biases in Perception and Cognition, 180–190 (Cambridge University Press, Cambridge, 2018).
LinkOut - more resources
Full Text Sources
Research Materials
