Nicotinic acetylcholine receptor-mediated effects of varenicline on LPS-elevated prostaglandin and cyclooxygenase levels in RAW 264.7 macrophages
- PMID: 38859932
- PMCID: PMC11163068
- DOI: 10.3389/fmolb.2024.1392689
Nicotinic acetylcholine receptor-mediated effects of varenicline on LPS-elevated prostaglandin and cyclooxygenase levels in RAW 264.7 macrophages
Abstract
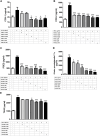
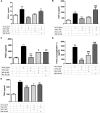
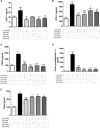
Introduction: The purpose of this study is to delineate anti-inflammatory and antioxidant potential of varenicline, a cigarette smoking cessation aid, on decreasing lipopolysaccharide (LPS)-elevated proinflammatory cytokines in RAW 264.7 murine macrophage cultures which we showed earlier to occur via cholinergic anti-inflammatory pathway (CAP) activation. To this end, we investigated the possible suppressive capacity of varenicline on LPS-regulated cyclooxygenase (COX-1 and COX-2) via α7 nicotinic acetylcholine receptor (α7nAChR) activation using the same in vitro model. Materials and Methods: In order to test anti-inflammatory effectiveness of varenicline, the levels of COX isoforms and products (PGE2, 6-keto PGF1α, a stable analog of PGI2, and TXA2) altered after LPS administration were determined by Enzyme Linked Immunosorbent Assay (ELISA). The antioxidant effects of varenicline were assessed by measuring reductions in reactive oxygen species (ROS) using a fluorometric intracellular ROS assay kit. We further investigated the contribution of nAChR subtypes by using non-selective and/or selective α7nAChR antagonists. The results were compared with that of conventional anti-inflammatory medications, such as ibuprofen, celecoxib and dexamethasone. Results: Varenicline significantly reduced LPS-induced COX-1, COX-2 and prostaglandin levels and ROS to an extent similar to that observed with anti-inflammatory agents used. Discussion: Significant downregulation in LPS-induced COX isoforms and associated decreases in PGE2, 6-keto PGF1α, and TXA2 levels along with reduction in ROS may be partly mediated via varenicline-activated α7nAChRs.
Keywords: cyclooxygenase; inflammation; prostaglandins; varenicline; α7nAChR.
Copyright © 2024 Baris, Arici and Tosun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Varenicline Prevents LPS-Induced Inflammatory Response via Nicotinic Acetylcholine Receptors in RAW 264.7 Macrophages.Front Mol Biosci. 2021 Oct 12;8:721533. doi: 10.3389/fmolb.2021.721533. eCollection 2021. Front Mol Biosci. 2021. PMID: 34712695 Free PMC article.
-
Liraglutide modulates cyclooxygenase and α7 acetylcholine receptors: in vitro and in silico insights into its anti-inflammatory role in LPS-induced inflammation in RAW 264.7 macrophages.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 31. doi: 10.1007/s00210-025-04225-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40448826
-
Activation of alpha7 nicotinic acetylcholine receptor protects bovine endometrial tissue against LPS-induced inflammatory injury via JAK2/STAT3 pathway and COX-2 derived prostaglandin E2.Eur J Pharmacol. 2021 Jun 5;900:174067. doi: 10.1016/j.ejphar.2021.174067. Epub 2021 Mar 31. Eur J Pharmacol. 2021. PMID: 33811838
-
Neuroimmune nexus in the pathophysiology and therapy of inflammatory disorders: Role of α7 nicotinic acetylcholine receptors.Pharmacol Res. 2023 May;191:106758. doi: 10.1016/j.phrs.2023.106758. Epub 2023 Apr 5. Pharmacol Res. 2023. PMID: 37028776 Review.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
Cited by
-
Nicotine-Induced Transient Activation of Monocytes Facilitates Immunosuppressive Macrophage Polarization that Restrains T Helper 17 Cell Expansion.Inflammation. 2025 Aug;48(4):2313-2322. doi: 10.1007/s10753-024-02191-3. Epub 2024 Nov 28. Inflammation. 2025. PMID: 39604662 Free PMC article.
-
Determination of nine prostaglandins in the arachidonic acid metabolic pathway with UHPLC-QQQ-MS/MS and application to in vitro and in vivo inflammation models.Front Pharmacol. 2025 May 30;16:1595059. doi: 10.3389/fphar.2025.1595059. eCollection 2025. Front Pharmacol. 2025. PMID: 40520189 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

