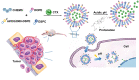
Design and Application of pH-Responsive Liposomes for Site-Specific Delivery of Cytotoxin from Cobra Venom
- PMID: 38859950
- PMCID: PMC11164093
- DOI: 10.2147/IJN.S461728
Design and Application of pH-Responsive Liposomes for Site-Specific Delivery of Cytotoxin from Cobra Venom
Abstract
Background: Current immunotherapies with unexpected severe side effects and treatment resistance have not resulted in the desired outcomes for patients with melanoma, and there is a need to discover more effective medications. Cytotoxin (CTX) from Cobra Venom has been established to have favorable cytolytic activity and antitumor efficacy and is regarded as a promising novel anticancer agent. However, amphiphilic CTX with excellent anionic phosphatidylserine lipid-binding ability may also damage normal cells.
Methods: We developed pH-responsive liposomes with a high CTX load (CTX@PSL) for targeted acidic-stimuli release of drugs in the tumor microenvironment. The morphology, size, zeta potential, drug-release kinetics, and preservation stability were characterized. Cell uptake, apoptosis-promoting effects, and cytotoxicity were assessed using MTT assay and flow cytometry. Finally, the tissue distribution and antitumor effects of CTX@PSL were systematically assessed using an in vivo imaging system.
Results: CTX@PSL exhibited high drug entrapment efficiency, drug loading, stability, and a rapid release profile under acidic conditions. These nanoparticles, irregularly spherical in shape and small in size, can effectively accumulate at tumor sites (six times higher than free CTX) and are rapidly internalized into cancer cells (2.5-fold higher cell uptake efficiency). CTX@PSL displayed significantly stronger cytotoxicity (IC50 0.25 μg/mL) and increased apoptosis in than the other formulations (apoptosis rate 71.78±1.70%). CTX@PSL showed considerably better tumor inhibition efficacy than free CTX or conventional liposomes (tumor inhibition rate 79.78±5.93%).
Conclusion: Our results suggest that CTX@PSL improves tumor-site accumulation and intracellular uptake for sustained and targeted CTX release. By combining the advantages of CTX and stimuli-responsive nanotechnology, the novel CTX@PSL nanoformulation is a promising therapeutic candidate for cancer treatment.
Keywords: cobra venom cytotoxin; liposomes; pH-responsive; targeted delivery; tumor microenvironment.
© 2024 Lin et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

