The Synergistic Effect of High Intensity Focused Ultrasound on In-vitro Remineralization of Tooth Enamel by Calcium Phosphate Ion Clusters
- PMID: 38859951
- PMCID: PMC11164203
- DOI: 10.2147/IJN.S464998
The Synergistic Effect of High Intensity Focused Ultrasound on In-vitro Remineralization of Tooth Enamel by Calcium Phosphate Ion Clusters
Abstract
Background: Remineralization of dental enamel is an important intervention strategy for the treatment of demineralized lesions. Existing approaches have limitations such as failure to adequately reproduce both the ideal structural and mechanical properties of the native tooth. The ability of ultrasound to control and accelerate the crystallization processes has been widely reported. Therefore, a new approach was explored for in-vitro enamel remineralization involving the synergistic effect of high-intensity focused ultrasound (HIFU) coupled with calcium phosphate ion clusters (CPICs).
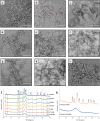
Methods: The demineralized enamel was treated with CPICs, with or without subsequent HIFU exposure for different periods (2.5, 5, and 10 min). The specimens were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), and Raman spectroscopy. The surface hardness and crystallographic properties of the treated specimens were evaluated using Vickers microhardness testing and X-ray diffraction (XRD), respectively.
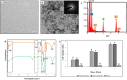
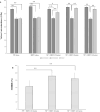
Results: SEM revealed distinct, organized, and well-defined prismatic structures, showing clear evidence of remineralization in the combined CPIC/HIFU treatment groups. AFM further revealed a decrease in the surface roughness values with increasing HIFU exposure time up to 5 min, reflecting the obliteration of interprismatic spaces created during demineralization. The characteristic Raman band at 960 cm-1 associated with the inorganic phase of enamel dominated well in the HIFU-treated specimens. Importantly, microhardness testing further demonstrated that new mineral growth also recovered the mechanical properties of the enamel in the HIFU-exposed groups. Critical to our aspirations for developing this into a clinical process, these results were achieved in only 5 min.
Conclusion: HIFU exposure can synergise and significantly accelerate in-vitro enamel remineralization process via calcium phosphate ion clusters. Therefore, this synergistic approach has the potential for use in future clinical interventions.
Keywords: calcium phosphate ion cluster; enamel remineralization; high intensity focused ultrasound.
© 2024 Shrestha et al.
Conflict of interest statement
Dr Barsha Shrestha reports grants from the National Health and Medical Research Council grant and grants from the Australian Dental Research Foundation grant, during the conduct of the study. Mrs Sheetal Maria Rajan reports grants from the National Health and Medical Research Council and grants from the Australian Dental Research Foundation, during the conduct of the study. Dr Sultan Aati reports grants from the National Health and Medical Research Council and grants from the Australian Dental Research Foundation, during the conduct of the study. Dr Emielda Yusiharni reports grants from the National Health and Medical Research Council grant (NMHRC, 1188401) and grants from the Australian Dental Research Foundation grant (ADRF; 0080045000900), during the conduct of the study. Dr Martin Saunders reports grants from the National Health and Medical Research Council and grants from the Australian Dental Research Foundation, during the conduct of the study. Dr. Amr Fawzy (lead chief investigator) reports grants from the National Health and Medical Research Council grant (NMHRC, 1188401) and grants from the Australian Dental Research Foundation grant (ADRF; 0080045000900), during the conduct of the study. The authors declare no other competing interests in this work.
Figures





Similar articles
-
Potential of High-Intensity Focused Ultrasound in Enamel Remineralization.J Dent Res. 2025 Aug;104(9):983-992. doi: 10.1177/00220345251323869. Epub 2025 Mar 19. J Dent Res. 2025. PMID: 40108508 Free PMC article.
-
Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions.J Dent. 2014 Apr;42(4):466-74. doi: 10.1016/j.jdent.2013.12.017. Epub 2014 Jan 9. J Dent. 2014. PMID: 24412586
-
In situ clinical effects of new dentifrices containing 1.5% arginine and fluoride on enamel de- and remineralization and plaque metabolism.J Clin Dent. 2013;24 Spec no A:A32-44. J Clin Dent. 2013. PMID: 24156138 Clinical Trial.
-
The Remineralization of Enamel from Saliva: A Chemical Perspective.Dent J (Basel). 2024 Oct 23;12(11):339. doi: 10.3390/dj12110339. Dent J (Basel). 2024. PMID: 39590389 Free PMC article. Review.
-
High-Intensity Focused Ultrasound in Dentistry: A Literature Review.Int Dent J. 2024 Oct;74(5):1168-1173. doi: 10.1016/j.identj.2024.02.004. Epub 2024 Apr 11. Int Dent J. 2024. PMID: 38609759 Free PMC article. Review.
Cited by
-
High-Intensity Focused Ultrasound Versus Airflow® in Debriding Ti-Attached S. mutans Biofilms.Int Dent J. 2025 Jun;75(3):1532-1543. doi: 10.1016/j.identj.2024.12.037. Epub 2025 Mar 22. Int Dent J. 2025. PMID: 40121850 Free PMC article.
-
Potential of High-Intensity Focused Ultrasound in Enamel Remineralization.J Dent Res. 2025 Aug;104(9):983-992. doi: 10.1177/00220345251323869. Epub 2025 Mar 19. J Dent Res. 2025. PMID: 40108508 Free PMC article.
References
-
- World Health Organization. Global oral health status report: towards universal health coverage for oral health by 2030.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

