Non-coding RNAs and exosomal non-coding RNAs in lung cancer: insights into their functions
- PMID: 38859962
- PMCID: PMC11163066
- DOI: 10.3389/fcell.2024.1397788
Non-coding RNAs and exosomal non-coding RNAs in lung cancer: insights into their functions
Abstract
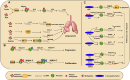
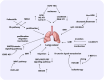
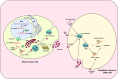
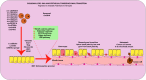
Lung cancer is the second most common form of cancer worldwide Research points to the pivotal role of non-coding RNAs (ncRNAs) in controlling and managing the pathology by controlling essential pathways. ncRNAs have all been identified as being either up- or downregulated among individuals suffering from lung cancer thus hinting that they may play a role in either promoting or suppressing the spread of the disease. Several ncRNAs could be effective non-invasive biomarkers to diagnose or even serve as effective treatment options for those with lung cancer, and several molecules have emerged as potential targets of interest. Given that ncRNAs are contained in exosomes and are implicated in the development and progression of the malady. Herein, we have summarized the role of ncRNAs in lung cancer. Moreover, we highlight the role of exosomal ncRNAs in lung cancer.
Keywords: circular RNA; long non-coding RNA; lung cancer; microRNA; non-coding RNA.
Copyright © 2024 Lv, Yang, Xie and Momeni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Current landscape of exosomal non-coding RNAs in prostate cancer: Modulators and biomarkers.Noncoding RNA Res. 2024 Jul 20;9(4):1351-1362. doi: 10.1016/j.ncrna.2024.07.003. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39247145 Free PMC article. Review.
-
Unveiling the role of ferroptosis-associated exosomal non-coding RNAs in cancer pathogenesis.Biomed Pharmacother. 2024 Mar;172:116235. doi: 10.1016/j.biopha.2024.116235. Epub 2024 Feb 3. Biomed Pharmacother. 2024. PMID: 38308967 Review.
-
The Potential Roles of Exosomal Non-Coding RNAs in Hepatocellular Carcinoma.Front Oncol. 2022 Feb 24;12:790916. doi: 10.3389/fonc.2022.790916. eCollection 2022. Front Oncol. 2022. PMID: 35280805 Free PMC article. Review.
-
Role of Exosomal Non-coding RNAs in Gastric Cancer: Biological Functions and Potential Clinical Applications.Front Oncol. 2021 Jun 14;11:700168. doi: 10.3389/fonc.2021.700168. eCollection 2021. Front Oncol. 2021. PMID: 34195097 Free PMC article. Review.
-
Exosomal ncRNAs in liquid biopsies for lung cancer.Clin Chim Acta. 2025 Jan 15;565:119983. doi: 10.1016/j.cca.2024.119983. Epub 2024 Oct 3. Clin Chim Acta. 2025. PMID: 39368685 Review.
Cited by
-
Identification of a Plasma Exosomal lncRNA- and circRNA-Based ceRNA Regulatory Network in Patients With Lung Adenocarcinoma.Clin Respir J. 2024 Oct;18(10):e70026. doi: 10.1111/crj.70026. Clin Respir J. 2024. PMID: 39428538 Free PMC article.
References
-
- Adi Harel S., Bossel Ben-Moshe N., Aylon Y., Bublik D. R., Moskovits N., Toperoff G., et al. (2015). Reactivation of epigenetically silenced miR-512 and miR-373 sensitizes lung cancer cells to cisplatin and restricts tumor growth. Cell Death Differ. 22 (8), 1328–1340. 10.1038/cdd.2014.221 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

