REPRESENTATION AND EXTRAPOLATION: EVIDENCE FROM CLINICAL TRIALS
- PMID: 38859982
- PMCID: PMC11164133
- DOI: 10.1093/qje/qjad036
REPRESENTATION AND EXTRAPOLATION: EVIDENCE FROM CLINICAL TRIALS
Abstract
This article examines the consequences and causes of low enrollment of Black patients in clinical trials. We develop a simple model of similarity-based extrapolation that predicts that evidence is more relevant for decision-making by physicians and patients when it is more representative of the group being treated. This generates the key result that the perceived benefit of a medicine for a group depends not only on the average benefit from a trial but also on the share of patients from that group who were enrolled in the trial. In survey experiments, we find that physicians who care for Black patients are more willing to prescribe drugs tested in representative samples, an effect substantial enough to close observed gaps in the prescribing rates of new medicines. Black patients update more on drug efficacy when the sample that the drug is tested on is more representative, reducing Black-white patient gaps in beliefs about whether the drug will work as described. Despite these benefits of representative data, our framework and evidence suggest that those who have benefited more from past medical breakthroughs are less costly to enroll in the present, leading to persistence in who is represented in the evidence base.
Keywords: D91; I12; I14; O31; O33.
Figures
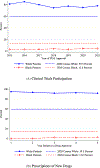
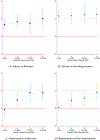
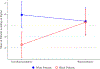
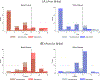

References
-
- Acemoglu Daron, and Linn Joshua, “Market Size in Innovation: Theory and Evidence from the Pharmaceutical Industry,” Quarterly Journal of Economics, 119 (2004), 1049–1090. 10.1162/0033553041502144 - DOI
-
- Agency for Healthcare Research and Quality, “MEPS Prescribed Medicine Files 1996–2019,” 2022, https://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp.
-
- Aghion Philippe, Akcigit Ufuk, Bergeaud Antonin, Blundell Richard, and Hémous David, “Innovation and Top Income Inequality,” Review of Economic Studies, 86 (2019), 1–45. 10.1093/restud/rdy027 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
