The effect of polyclonal and monoclonal based antibodies as promising potential therapy for treatment of sepsis: A systematic review
- PMID: 38860003
- PMCID: PMC11163170
- DOI: 10.1016/j.nmni.2024.101435
The effect of polyclonal and monoclonal based antibodies as promising potential therapy for treatment of sepsis: A systematic review
Abstract
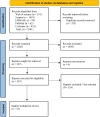
While mortality caused by sepsis remains an unsolved problem, studies showed conflicting results about effectiveness of monoclonal and polyclonal antibodies in patients suffering sepsis. For this reason, this current study provides an update of review clinical randomized trial studies until March 2024. The main object of this study is to determine effects of monoclonal and polyclonal antibodies on mortality rate and hospitalization of patients suffering sepsis. Search of Scopus, Web of science, EMBASE, PubMed and Cochrane were performed and randomized controlled trials which conducted in patients with septic shock or bacterial sepsis were included. Two reviewers assessed all searched trials for eligibility according to already defined criteria and did data collection and analyses afterwards. Present study showed monoclonal and polyclonal antibodies are a safe strategy with mild-to-moderate adverse effects. However, most studies indicate no significant change among inter-and intra-group comparison (p > 0.05) and further studies are needed, results showed an increase in survival rate, ventilator-and ICU-free days, resolve organ dysfunction, mediating inflammation related cytokines.
Keywords: Monoclonal antibody; Randomized clinical trial; Sepsis; Septic shock; Septicemia.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
EXCHANGE-2: investigating the efficacy of add-on plasma exchange as an adjunctive strategy against septic shock-a study protocol for a randomized, prospective, multicenter, open-label, controlled, parallel-group trial.Trials. 2023 Apr 15;24(1):277. doi: 10.1186/s13063-023-07300-5. Trials. 2023. PMID: 37061693 Free PMC article.
-
Thiamine, Ascorbic Acid, and Hydrocortisone As a Metabolic Resuscitation Cocktail in Sepsis: A Meta-Analysis of Randomized Controlled Trials With Trial Sequential Analysis.Crit Care Med. 2021 Dec 1;49(12):2112-2120. doi: 10.1097/CCM.0000000000005262. Crit Care Med. 2021. PMID: 34582409
-
Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis.Crit Care Med. 2007 Dec;35(12):2686-92. Crit Care Med. 2007. PMID: 18074465 Review.
-
The outcome of IV vitamin C therapy in patients with sepsis or septic shock: a meta-analysis of randomized controlled trials.Crit Care. 2023 Mar 13;27(1):109. doi: 10.1186/s13054-023-04392-y. Crit Care. 2023. PMID: 36915173 Free PMC article.
-
Efficacy and Safety of Vilobelimab (IFX-1), a Novel Monoclonal Anti-C5a Antibody, in Patients With Early Severe Sepsis or Septic Shock-A Randomized, Placebo-Controlled, Double-Blind, Multicenter, Phase IIa Trial (SCIENS Study).Crit Care Explor. 2021 Nov 17;3(11):e0577. doi: 10.1097/CCE.0000000000000577. eCollection 2021 Nov. Crit Care Explor. 2021. PMID: 34806021 Free PMC article.
References
-
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. - PubMed
-
- van der Poll T., Shankar-Hari M., Wiersinga W.J. The immunology of sepsis. Immunity. 2021;54(11):2450–2464. - PubMed
-
- Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. - PubMed
-
- Font M.D., Thyagarajan B., Khanna A.K. Sepsis and Septic Shock – basics of diagnosis, pathophysiology and clinical decision making. Med Clin. 2020;104(4):573–585. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
