Benralizumab Reduces Respiratory Exacerbations and Oral Glucocorticosteroid Dose in Patients with Severe Asthma and Eosinophilic Granulomatosis with Polyangiitis
- PMID: 38860030
- PMCID: PMC11164095
- DOI: 10.2147/JAA.S461800
Benralizumab Reduces Respiratory Exacerbations and Oral Glucocorticosteroid Dose in Patients with Severe Asthma and Eosinophilic Granulomatosis with Polyangiitis
Abstract
Background: Benralizumab reduces exacerbations and long-term oral glucocorticosteroid (OCS) exposure in patients with severe eosinophilic asthma. In patients with eosinophilic granulomatosis with polyangiitis (EGPA), uncontrolled symptoms and exacerbations of asthma and chronic rhinosinusitis (CRS) are important reasons for continued OCS therapies. We aimed to describe outcomes of patients with severe asthma and EGPA treated with benralizumab in real-life.
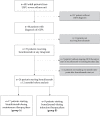
Methods: We retrospectively analyzed adult patients from the Severe Asthma Unit at LMU Munich diagnosed with severe asthma and EGPA treated with benralizumab, differentiating two groups: Group A, patients with a stable daily OCS dose and diagnosis of EGPA >6 months ago; and Group B, patients treated with high-dose daily OCS due to recent diagnosis of EGPA <6 months ago. We compared outcome parameters at baseline and 12 months after initiation of benralizumab, including respiratory exacerbations, daily OCS dose, and lung function.
Results: Group A included 17 patients, all receiving OCS therapy and additional immunosuppressants; 15 patients (88%) continued benralizumab for more than 12 months, demonstrating a significant reduction in daily OCS dose and exacerbations while FEV1 increased. Group B included 9 patients, all with high-dose daily OCS and some receiving cyclophosphamide pulse therapy for life-threatening disease. Benralizumab addition during induction was well tolerated. A total of 7/9 (78%) continued benralizumab for more than 12 months and preserved EGPA remission at the 12-month timepoint.
Conclusion: In this real-life cohort of patients with severe asthma and EGPA, benralizumab initiation during remission maintenance reduced respiratory exacerbations and daily OCS dose. Benralizumab initiation during remission induction was associated with a high rate of clinical EGPA remission.
Keywords: CRS; EGPA; OCS; anti-IL5R; asthma; benralizumab; biologics; glucocorticoid; vasculitis.
© 2024 Mümmler et al.
Conflict of interest statement
CM reports speaker and/or consultancy fees from Sanofi, outside of the submitted work. PM reports speaker and/or consultancy fees from AstraZeneca, ResMed, Insmed, Vertex, all outside of the submitted work. US has received consulting fees from Ablynx, Alexion, and Vifor Pharma and reports research support from Vifor Pharma, Ablynx/Sanofi, and Alexion, all outside the submitted work. UG received speaker honoraria from AstraZeneca outside of the submitted work. HSK served as consultant and advisor or review panel member for AbbVie, Amgen, AstraZeneca, Biogen, BMS, Celgene, Gilead, Galapagos, Hexal Sandoz, Janssen-Cilag, Elli Lilly, Medac, MSD, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, and UCB, all outside the submitted work. JB reports speaker and/or consultancy fees from AstraZeneca, GSK, Novartis, Sanofi, all outside of the submitted work. NK reports speaker and/or consultancy fees from AstraZeneca and GSK, all outside of the submitted work. KM reports speaker and/or consultancy fees from AstraZeneca, Chiesi, GSK, Novartis, Sanofi, all outside of the submitted work. The author report no other conflicts of interest in this work.
Figures


Similar articles
-
Effectiveness and safety of anti-IL-5/Rα biologics in eosinophilic granulomatosis with polyangiitis: a two-year multicenter observational study.Front Immunol. 2023 Jun 30;14:1204444. doi: 10.3389/fimmu.2023.1204444. eCollection 2023. Front Immunol. 2023. PMID: 37457743 Free PMC article.
-
Long-Term Effectiveness of Benralizumab in Eosinophilic Granulomatosis With Polyangiitis.J Allergy Clin Immunol Pract. 2024 Mar;12(3):724-732. doi: 10.1016/j.jaip.2024.01.006. Epub 2024 Jan 9. J Allergy Clin Immunol Pract. 2024. PMID: 38211889
-
Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis.J Allergy Clin Immunol Pract. 2021 Mar;9(3):1186-1193.e1. doi: 10.1016/j.jaip.2020.09.054. Epub 2020 Oct 14. J Allergy Clin Immunol Pract. 2021. PMID: 33065367 Clinical Trial.
-
Perspectives on the Efficacy of Benralizumab for Treatment of Eosinophilic Granulomatosis With Polyangiitis.Front Pharmacol. 2022 Mar 10;13:865318. doi: 10.3389/fphar.2022.865318. eCollection 2022. Front Pharmacol. 2022. PMID: 35359852 Free PMC article. Review.
-
Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma.Respir Med. 2019 Apr;150:51-62. doi: 10.1016/j.rmed.2019.02.003. Epub 2019 Feb 7. Respir Med. 2019. PMID: 30961951 Review.
References
-
- Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French vasculitis study group cohort. Arthritis Rheum. 2013;65(1):270–281. doi:10.1002/art.37721 - DOI - PubMed
LinkOut - more resources
Full Text Sources

