Computational analysis of cancer cell adhesion in curved vessels affected by wall shear stress for prediction of metastatic spreading
- PMID: 38860135
- PMCID: PMC11163055
- DOI: 10.3389/fbioe.2024.1393413
Computational analysis of cancer cell adhesion in curved vessels affected by wall shear stress for prediction of metastatic spreading
Abstract
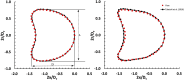
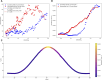
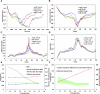
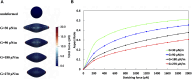
Introduction: The dynamics of circulating tumor cells (CTCs) within blood vessels play a pivotal role in predicting metastatic spreading of cancer within the body. However, the limited understanding and method to quantitatively investigate the influence of vascular architecture on CTC dynamics hinders our ability to predict metastatic process effectively. To address this limitation, the present study was conducted to investigate the influence of blood vessel tortuosity on the behaviour of CTCs, focusing specifically on establishing methods and examining the role of shear stress in CTC-vessel wall interactions and its subsequent impact on metastasis. Methods: We computationally simulated CTC behaviour under various shear stress conditions induced by vessel tortuosity. Our computational model, based on the lattice Boltzmann method (LBM) and a coarse-grained spectrin-link membrane model, efficiently simulates blood plasma dynamics and CTC deformability. The model incorporates fluid-structure interactions and receptor-ligand interactions crucial for CTC adhesion using the immersed boundary method (IBM). Results: Our findings reveal that uniform shear stress in straight vessels leads to predictable CTC-vessel interactions, whereas in curved vessels, asymmetrical flow patterns and altered shear stress create distinct adhesion dynamics, potentially influencing CTC extravasation. Quantitative analysis shows a 25% decrease in the wall shear stress in low-shear regions and a 58.5% increase in the high-shear region. We observed high-shear regions in curved vessels to be potential sites for increased CTC adhesion and extravasation, facilitated by elevated endothelial expression of adhesion molecules. This phenomenon correlates with the increased number of adhesion bonds, which rises to approximately 40 in high-shear regions, compared to around 12 for straight vessels and approximately 5-6 in low-shear regions. The findings also indicate an optimal cellular stiffness necessary for successful CTC extravasation in curved vessels. Discussion: By the quantitative assessment of the risk of CTC extravasation as a function of vessel tortuosity, our study offers a novel tool for the prediction of metastasis risk to support the development of personalized therapeutic interventions based on individual vascular characteristics and tumor cell properties.
Keywords: cancer models; cell adhesion; computational biophysics; metastasis; microvessel configuration.
Copyright © 2024 Rahmati and Maftoon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Effects of wall shear stress and its gradient on tumor cell adhesion in curved microvessels.Biomech Model Mechanobiol. 2012 May;11(5):641-53. doi: 10.1007/s10237-011-0339-6. Epub 2011 Aug 5. Biomech Model Mechanobiol. 2012. PMID: 21818636 Free PMC article.
-
Investigating the Interaction Between Circulating Tumor Cells and Local Hydrodynamics via Experiment and Simulations.Cell Mol Bioeng. 2020 Oct 21;13(5):527-540. doi: 10.1007/s12195-020-00656-7. eCollection 2020 Oct. Cell Mol Bioeng. 2020. PMID: 33184581 Free PMC article.
-
Numerical study on the adhesion of a circulating tumor cell in a curved microvessel.Biomech Model Mechanobiol. 2021 Feb;20(1):243-254. doi: 10.1007/s10237-020-01380-x. Epub 2020 Aug 18. Biomech Model Mechanobiol. 2021. PMID: 32809129
-
Modeling Cell Adhesion and Extravasation in Microvascular System.Adv Exp Med Biol. 2018;1097:219-234. doi: 10.1007/978-3-319-96445-4_12. Adv Exp Med Biol. 2018. PMID: 30315548 Review.
-
Beyond Enumeration: Functional and Computational Analysis of Circulating Tumor Cells to Investigate Cancer Metastasis.Front Med (Lausanne). 2018 Feb 19;5:34. doi: 10.3389/fmed.2018.00034. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29520361 Free PMC article. Review.
Cited by
-
Intravasation-On-µDevice (INVADE): Engineering Dynamic Vascular Interfaces to Study Cancer Cell Intravasation.Adv Mater. 2025 Jul;37(26):e2501466. doi: 10.1002/adma.202501466. Epub 2025 Apr 13. Adv Mater. 2025. PMID: 40223399 Free PMC article.
-
Circulating Tumor Cells: Origin, Role, Current Applications, and Future Perspectives for Personalized Medicine.Biomedicines. 2024 Sep 20;12(9):2137. doi: 10.3390/biomedicines12092137. Biomedicines. 2024. PMID: 39335650 Free PMC article. Review.
References
-
- Anvari S., Nambiar S., Pang J., Maftoon N. (2021). Computational models and simulations of cancer metastasis. Arch. Comput. Methods Eng. 28, 4837–4859. 10.1007/s11831-021-09554-1 - DOI
-
- Arabghahestani M., Poozesh S., Akafuah N. K. (2019). Advances in computational fluid mechanics in cellular flow manipulation: a review. Appl. Sci. 9, 4041. 10.3390/app9194041 - DOI
-
- Azizi Tarksalooyeh V. W., Závodszky G., van Rooij J. M., Hoekstra A. G. (2018). Inflow and outflow boundary conditions for 2D suspension simulations with the immersed boundary lattice Boltzmann method. Comput. Fluids 172, 312–317. 10.1016/j.compfluid.2018.04.025 - DOI
LinkOut - more resources
Full Text Sources

