Phytochemical analysis, physicochemical, pharmacokinetic properties and molecular docking studies of bioactive compounds in Ottelia alismoides (L.) pers. Against breast cancer proteins
- PMID: 38860144
- PMCID: PMC11162403
- DOI: 10.1007/s40203-024-00227-y
Phytochemical analysis, physicochemical, pharmacokinetic properties and molecular docking studies of bioactive compounds in Ottelia alismoides (L.) pers. Against breast cancer proteins
Abstract
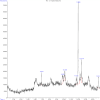
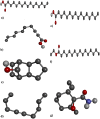
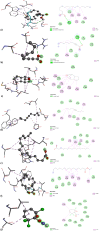
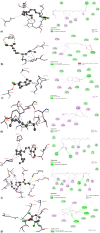
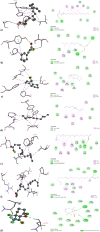
Plants provide compounds that can be used to treat diseases, and in silico methods help to expedite drug discovery while reducing costs. This study explored the phytochemical profile of methanol extract of O. alismoides using GC-MS to identify potential bioactive compounds. Autodock 4.2.6. was employed for molecular docking evaluation of the efficacy of these identified compounds against Estrogen Receptor Alpha (ERα), Human Epidermal Growth Factor Receptor 2 (HER2), and Epidermal Growth Factor Receptor (EGFR), proteins. Additionally, the ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties of the compounds were predicted using the SwissADME online tool. The preliminary phytochemical analysis revealed the presence of alkaloids, carbohydrates, glycosides, and steroids. During the GC-MS analysis, seven compounds were identified, and drug-likeness prediction of these compounds showed good pharmacokinetic properties having high gastrointestinal absorption, and orally bioavailable. The molecular docking studies exhibited promising binding affinities of bioactive compounds against all target proteins. Specifically, the compounds Tricyclo[5.2.1.0(2,6)]decan-10-ol and 2,2,6-Trichloro-7-oxabicyclo[4.1.0]heptane-1-carboxamide demonstrated the highest binding affinities with the ERα (-6.3 and - 6.0 k/cal), HER2 (-5.6 and - 6.1 k/cal), and EGFR (-5.4 and - 5.4 k/cal), respectively. These findings suggest the potential of O. alismoides as a source for developing new cancer therapeutics. The study highlights the effectiveness of in silico approaches for accelerating drug discovery from natural sources and paves the way for further exploration of these promising compounds.
Supplementary information: The online version contains supplementary material available at 10.1007/s40203-024-00227-y.
Keywords: Ethyl palmitate and drug-likeness properties; Molecular Docking; Ottelia alismoides; Palmitic acid.
© The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no competing interests.
Figures
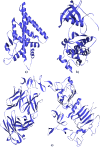
Similar articles
-
Chemical Characterization, In-silico Evaluation, and Molecular Docking Analysis of Antiproliferative Compounds Isolated from the Bark of Anthocephalus cadamba Miq.Anticancer Agents Med Chem. 2022;22(20):3416-3437. doi: 10.2174/1871520622666220204123348. Anticancer Agents Med Chem. 2022. PMID: 35125087
-
Effect of acetone fraction of Ottelia alismoides on the G2/M cell cycle arrest and apoptosis in the human carcinoma cell lines.J Ethnopharmacol. 2023 Jan 10;300:115729. doi: 10.1016/j.jep.2022.115729. Epub 2022 Sep 24. J Ethnopharmacol. 2023. PMID: 36162544
-
Estimation of drug-likeness properties of GC-MS separated bioactive compounds in rare medicinal Pleione maculata using molecular docking technique and SwissADME in silico tools.Netw Model Anal Health Inform Bioinform. 2021;10(1):14. doi: 10.1007/s13721-020-00276-1. Epub 2021 Feb 24. Netw Model Anal Health Inform Bioinform. 2021. PMID: 33643765 Free PMC article.
-
In silico evaluation of potential breast cancer receptor antagonists from GC-MS and HPLC identified compounds in Pleurotus ostreatus extracts.RSC Adv. 2024 Aug 9;14(33):23744-23771. doi: 10.1039/d4ra03832k. eCollection 2024 Jul 26. RSC Adv. 2024. PMID: 39131188 Free PMC article.
-
Phytochemical Characterization, Functional Nutrition, and Anti-Diabetic Potentials of Leptadenia hastata (pers) Decne Leaves: In Silico and In Vitro Studies.Bioinform Biol Insights. 2022 Aug 11;16:11779322221115436. doi: 10.1177/11779322221115436. eCollection 2022. Bioinform Biol Insights. 2022. PMID: 35982736 Free PMC article.
References
-
- Al-Zahrani A, Ibraheem F, El-Senduny FF (2022) Anticancer activities of Saudi Juniperus procera extracts and their effects on the regulatory mechanisms in controlling growth of human cancerous cells. Res Sq 1–25. 10.21203/rs.3.rs-1666736/v1
-
- Banu KS, Cathrine L. General techniques involved in phytochemical analysis. Int j adv res chem sci. 2015;2(4):25–32.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

