N-heterocyclic carbene catalyzed [2 + 3] annulation reaction for the synthesis of trifluoroethyl 3,2'-spirooxindole γ-lactam
- PMID: 38860250
- PMCID: PMC11163332
- DOI: 10.1039/d4ra02252a
N-heterocyclic carbene catalyzed [2 + 3] annulation reaction for the synthesis of trifluoroethyl 3,2'-spirooxindole γ-lactam
Abstract
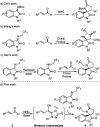
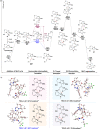
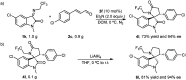
Asymmetric catalytic processes promoted by N-heterocyclic carbenes (NHCs) hold great potential for the sustainable preparation of chiral molecules. However, catalyzing the reactions by manipulating the reactive intermediates is challenging. We report herein that the known NHC-catalyzed [3 + 2] annulation reaction between ketimine and enal can also be turned into a [2 + 3] annulation reaction for the highly enantioselective direct synthesis of trifluoroethyl 3,2'-spirooxindole γ-lactams (4) through timely catalysis of the intermediates. DFT calculations revealed that this transformation included the key step of the nucleophilic attack of the Breslow intermediate M2 derived from NHC and enal (2) to the unattacked ketimine (1). Our study demonstrates that it is possible to tune the desired selectivities through the dynamic catalysts of the reactive intermediates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
-
N-Heterocyclic Carbene (NHC)-Catalyzed Transformations Involving Azolium Enolates.Chem Rec. 2022 Aug;22(8):e202200054. doi: 10.1002/tcr.202200054. Epub 2022 May 13. Chem Rec. 2022. PMID: 35562645 Review.
-
On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums.Acc Chem Res. 2014 Feb 18;47(2):696-707. doi: 10.1021/ar400239v. Epub 2014 Jan 10. Acc Chem Res. 2014. PMID: 24410291
-
Enantioselective [3+3] atroposelective annulation catalyzed by N-heterocyclic carbenes.Nat Commun. 2018 Feb 9;9(1):611. doi: 10.1038/s41467-018-02952-3. Nat Commun. 2018. PMID: 29426851 Free PMC article.
-
Enantioselective Synthesis of Spirocyclohexadienones by NHC-Catalyzed Formal [3+3] Annulation Reaction of Enals.Angew Chem Int Ed Engl. 2016 Jan 4;55(1):268-72. doi: 10.1002/anie.201507802. Epub 2015 Oct 21. Angew Chem Int Ed Engl. 2016. PMID: 26487242
References
-
- Gras E. and Chassaing S., Carbenes and Nitrenes, Wiley Online Library, The United States of America, 2019, pp. 219–249
-
- Ronald B. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958;80:3719–3726.
-
- Mondal S. Ghosh A. Biju A. T. N. Heterocyclic Carbene (NHC)-Catalyzed Transformations Involving Azolium Enolates. Chem. Rec. 2022;22:e202200054. - PubMed
-
- Sohn S. S. Rosen E. L. Bode J. W. N-heterocyclic carbene-catalyzed generation of homoenolates: gamma-butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc. 2004;126:14370–14371. - PubMed
- Nair V. Vellalath S. Poonoth M. Mohan R. Suresh E. N. heterocyclic carbene catalyzed reaction of enals and 1,2-dicarbonyl compounds: stereoselective synthesis of spiro gamma-butyrolactones. Org. Lett. 2006;8:507–509. - PubMed
- Rommel M. Fukuzumi T. Bode J. W. Cyclic ketimines as superior electrophiles for NHC-catalyzed homoenolate additions with broad scope and low catalyst loadings. J. Am. Chem. Soc. 2008;130:17266–17267. - PMC - PubMed
- Sun L. H. Shen L. T. Ye S. Highly diastereo- and enantioselective NHC-catalyzed [3+2] annulation of enals and isatins. Chem. Commun. 2011;47:10136–10138. - PubMed
- Dugal-Tessier J. O'Bryan E. A. Schroeder T. B. Cohen D. T. Scheidt K. A. An N-heterocyclic carbene/Lewis acid strategy for the stereoselective synthesis of spirooxindole lactones. Angew Chem. Int. Ed. Engl. 2012;51:4963–4967. - PMC - PubMed
- Menon R. S. Biju A. T. Nair V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon-carbon bond-forming reactions. Chem. Soc. Rev. 2015;44:5040–5052. - PubMed
- Mukherjee S. Joseph S. Bhunia A. Gonnade R. G. Yetra S. R. Biju A. T. Enantioselective synthesis of spiro γ-butyrolactones by N-heterocyclic carbene (NHC)-catalyzed formal [3 + 2] annulation of enals with 3-hydroxy oxindoles. Org. Biomol. Chem. 2017;15:2013–2019. - PubMed
-
- Mondal S. Yetra S. R. Mukherjee S. Biju A. T. NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles. Acc. Chem. Res. 2019;52:425–436. - PubMed
LinkOut - more resources
Full Text Sources

