FXR deletion attenuates intestinal barrier dysfunction in murine acute intestinal inflammation
- PMID: 38860296
- PMCID: PMC11427094
- DOI: 10.1152/ajpgi.00063.2024
FXR deletion attenuates intestinal barrier dysfunction in murine acute intestinal inflammation
Abstract
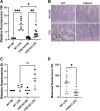
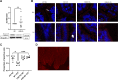
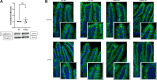
Accumulating literature suggests that the farnesoid-X receptor (FXR), a nuclear bile acid receptor best known for its role in bile acid homeostasis, is also a potent context-dependent regulator of inflammation. FXR may thus be relevant to several intestinal disease states including inflammatory bowel disease, necrotizing enterocolitis, and sepsis. In this study, we tested the effects of FXR deletion on acute murine intestinal inflammation. We found that FXR knockout (KO) mice were protected from intestinal injury and barrier dysfunction induced by lipopolysaccharide (LPS) injection, dithizone (DI)/Klebsiella, and cecal ligation/puncture models. In the LPS model, RNA sequencing and qPCR analysis showed that this protection correlated with substantial reduction in LPS-induced proinflammatory gene expression, including lower tissue levels of Il1a, Il1b, and Tnf. Examining functional effects on the epithelium, we found that LPS-induced tight junctional disruption as assessed by internalization of ZO-1 and occludin was ameliorated in FXR KO animals. Taken together, these data suggest a role for FXR in the intestinal barrier during inflammatory injury.NEW & NOTEWORTHY Intestinal barrier failure is a hallmark in gut-origin sepsis. We demonstrate that the intestinal barriers of farnesoid-X receptor (FXR) knockout (KO) animals are protected from inflammatory insult using multiple models of acute intestinal inflammation. This protection is due to decreased inflammatory cytokine production and maintenance of tight junctional architecture seen within the KO animals. This is the first report of FXR deletion being protective to the intestinal barrier.
Keywords: FXR; LPS; inflammation; intestinal barrier; tight junctions.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







Comment in
-
Regional and conditional variability of FXR: new lessons on ileal inflammation and gut barrier functions.Am J Physiol Gastrointest Liver Physiol. 2024 Nov 1;327(5):G626-G628. doi: 10.1152/ajpgi.00226.2024. Epub 2024 Aug 27. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39189790 No abstract available.
Similar articles
-
Farnesoid X receptor inhibits proinflammatory cytokine-induced epithelial necroptosis in vitro: implications for preservation of intestinal barrier function.Am J Physiol Gastrointest Liver Physiol. 2025 Aug 1;329(2):G261-G269. doi: 10.1152/ajpgi.00086.2025. Epub 2025 Jun 26. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40569574
-
Investigating intestinal farnesoid X receptor functions at the intestinal mucosal barrier and in the intestinal microbiota in a biliary obstruction mouse model.Am J Physiol Gastrointest Liver Physiol. 2025 Aug 1;329(2):G313-G327. doi: 10.1152/ajpgi.00223.2024. Epub 2025 Jul 7. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40623002
-
Sub-chronic realgar exposure causes liver inflammatory injury in mice by inducing bile acid-mediated NLRP3 inflammasome activation through down-regulation of ileal FXR.J Ethnopharmacol. 2025 Jul 24;351:120174. doi: 10.1016/j.jep.2025.120174. Epub 2025 Jun 18. J Ethnopharmacol. 2025. PMID: 40541751
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Bile acids affect intestinal barrier function through FXR and TGR5.Front Med (Lausanne). 2025 Jul 7;12:1607899. doi: 10.3389/fmed.2025.1607899. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40692955 Free PMC article. Review.
-
Advances of exosome regulating‑FXR to repair inflammatory bowel disease (Review).Int J Mol Med. 2025 Sep;56(3):135. doi: 10.3892/ijmm.2025.5576. Epub 2025 Jul 4. Int J Mol Med. 2025. PMID: 40613233 Free PMC article. Review.
-
Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment.Biomedicines. 2024 Dec 20;12(12):2910. doi: 10.3390/biomedicines12122910. Biomedicines. 2024. PMID: 39767816 Free PMC article. Review.
References
-
- Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182: 375–387, 2013. doi: 10.1016/j.ajpath.2012.10.014. - DOI - PMC - PubMed
-
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6: 498–510, 2013. doi: 10.1038/mi.2012.89. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

