Astragaloside IV inhibits inflammation caused by influenza virus via reactive oxygen species/NOD-like receptor thermal protein domain associated protein 3/Caspase-1 signaling pathway
- PMID: 38860765
- PMCID: PMC11165686
- DOI: 10.1002/iid3.1309
Astragaloside IV inhibits inflammation caused by influenza virus via reactive oxygen species/NOD-like receptor thermal protein domain associated protein 3/Caspase-1 signaling pathway
Abstract
Background: Astragaloside IV (AS-IV) is the most active monomer in the traditional Chinese herbal medicine Radix Astragali, which has a wide range of antiviral, anti-inflammatory, and antifibrosis pharmacological effects, and shows protective effects in acute lung injury.
Methods: This study utilized the immunofluorescence, flow cytometry, enzyme-linked immunosorbent assay, quantitative reverse transcription-polymerase chain reaction, western blot, and hematoxylin and eosin staining methods to investigate the mechanism of AS-IV in reducing viral pneumonia caused by influenza A virus in A549 cells and BALB/c mice.
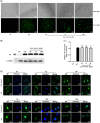
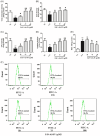
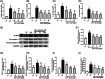
Results: The results showed that AS-IV suppressed reactive oxygen species production in influenza virus-infected A549 cells in a dose-dependent manner, and subsequently inhibited the activation of nucleotide-binding oligomerization domain-like receptor thermal protein domain associated protein 3 inflammasome and Caspase-1, decreased interleukin (IL) -1β and IL-18 secretion. In BALB/c mice infected with Poly (I:C), oral administration of AS-IV can significantly reduce Poly (I:C)-induced acute pneumonia and lung pathological injury.
Conclusions: AS-IV alleviates the inflammatory response induced by influenza virus in vitro and lung flammation and structural damage caused by poly (I:C) in vivo.
Keywords: Caspase‐1; NOD‐like receptor thermal protein domain associated protein 3; astragaloside IV; inflammation; influenza virus; reactive oxygen species.
© 2024 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Jinxin oral liquid reduced lung inflammation in influenza A virus infected mice through inhibiting NOD-like receptor protein 3 pathway.J Tradit Chin Med. 2025 Apr;45(2):281-290. doi: 10.19852/j.cnki.jtcm.2025.02.022. J Tradit Chin Med. 2025. PMID: 40151115 Free PMC article.
-
Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo.J Ethnopharmacol. 2020 Apr 24;252:112584. doi: 10.1016/j.jep.2020.112584. Epub 2020 Jan 21. J Ethnopharmacol. 2020. PMID: 31972325
-
Jinxin oral liquid inhibits human respiratory syncytial virus-induced excessive inflammation associated with blockade of the NLRP3/ASC/Caspase-1 pathway.Biomed Pharmacother. 2018 Jul;103:1376-1383. doi: 10.1016/j.biopha.2018.04.174. Epub 2018 May 7. Biomed Pharmacother. 2018. PMID: 29864921
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Astragaloside IV regulates the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to inhibit PM2.5-mediated lung injury in mice.Int Immunopharmacol. 2022 Nov;112:109186. doi: 10.1016/j.intimp.2022.109186. Epub 2022 Sep 15. Int Immunopharmacol. 2022. PMID: 36115280 Review.
Cited by
-
Saxifraga stolonifera inhibits porcine epidemic diarrhea virus infection by disrupting nucleocapsid protein-p53 interaction.Front Cell Infect Microbiol. 2025 Jul 9;15:1615300. doi: 10.3389/fcimb.2025.1615300. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40703674 Free PMC article.
-
Unveiling the intersection: ferroptosis in influenza virus infection.Virol J. 2024 Aug 12;21(1):185. doi: 10.1186/s12985-024-02462-3. Virol J. 2024. PMID: 39135112 Free PMC article. Review.
-
Astragaloside IV mitigates influenza-induced inflammatory responses by suppressing the Wnt/β-catenin signalling pathway in alveolar macrophages.Vet Res. 2025 Apr 30;56(1):95. doi: 10.1186/s13567-025-01529-5. Vet Res. 2025. PMID: 40307893 Free PMC article.
-
Antiviral treatment for viral pneumonia: current drugs and natural compounds.Virol J. 2025 Mar 6;22(1):62. doi: 10.1186/s12985-025-02666-1. Virol J. 2025. PMID: 40050867 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

