Polymerase Chain Reaction on Respiratory Tract Specimens of Immunocompromised Patients to Diagnose Pneumocystis Pneumonia: A Systematic Review and Meta-analysis
- PMID: 38860786
- PMCID: PMC11259226
- DOI: 10.1093/cid/ciae239
Polymerase Chain Reaction on Respiratory Tract Specimens of Immunocompromised Patients to Diagnose Pneumocystis Pneumonia: A Systematic Review and Meta-analysis
Abstract
Background: This meta-analysis examines the comparative diagnostic performance of polymerase chain reaction (PCR) for the diagnosis of Pneumocystis pneumonia (PCP) on different respiratory tract samples, in both human immunodeficiency virus (HIV) and non-HIV populations.
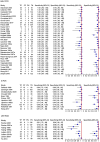
Methods: A total of 55 articles met inclusion criteria, including 11 434 PCR assays on respiratory specimens from 7835 patients at risk of PCP. QUADAS-2 tool indicated low risk of bias across all studies. Using a bivariate and random-effects meta-regression analysis, the diagnostic performance of PCR against the European Organisation for Research and Treatment of Cancer-Mycoses Study Group definition of proven PCP was examined.
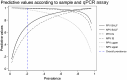
Results: Quantitative PCR (qPCR) on bronchoalveolar lavage fluid provided the highest pooled sensitivity of 98.7% (95% confidence interval [CI], 96.8%-99.5%), adequate specificity of 89.3% (95% CI, 84.4%-92.7%), negative likelihood ratio (LR-) of 0.014, and positive likelihood ratio (LR+) of 9.19. qPCR on induced sputum provided similarly high sensitivity of 99.0% (95% CI, 94.4%-99.3%) but a reduced specificity of 81.5% (95% CI, 72.1%-88.3%), LR- of 0.024, and LR+ of 5.30. qPCR on upper respiratory tract samples provided lower sensitivity of 89.2% (95% CI, 71.0%-96.5%), high specificity of 90.5% (95% CI, 80.9%-95.5%), LR- of 0.120, and LR+ of 9.34. There was no significant difference in sensitivity and specificity of PCR according to HIV status of patients.
Conclusions: On deeper respiratory tract specimens, PCR negativity can be used to confidently exclude PCP, but PCR positivity will likely require clinical interpretation to distinguish between colonization and active infection, partially dependent on the strength of the PCR signal (indicative of fungal burden), the specimen type, and patient population tested.
Keywords: PCP; meta-analysis; pneumocystis; polymerase chain reaction; systematic review.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. A. has received honoraria from Gilead Sciences. S. C.-A. A. has received united educational grants from F2G Ltd and consulted for MSD Australia. T. R. R. has received grants from Pfizer and the Irish Department of Agriculture, Food and the Marine and honoraria from Gilead and Menarini Pharma. P. L. W. has performed diagnostic evaluations and received meeting sponsorship from Associates of Cape Cod, Bruker, Dynamiker, and Launch Diagnostics; speaker fees, expert advice fees, and meeting sponsorship from Gilead; and speaker and expert advice fees from F2G. J. P. D. has received personal fees from F2G, Gilead Sciences, and Pfizer. W. J. H. has received lecture honoraria from AbbVie, Amgen, AstraZeneca, Celgene/BMS, Gilead Sciences, Janssen, MSD, and Pfizer; has received support to attend meetings and travel support from AbbVie, Alexion, Astellas, Janssen, Lilly, MSD, Novartis, and Pfizer; and has participated on advisory boards for AbbVie, Amgen, AstraZeneca, Basilea, Celgene/BMS, Gilead Sciences, Janssen, and Sanofi-Aventis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Pegorie M, Denning DW, Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect 2017; 74:60–71. - PubMed
-
- Cordonnier C, Cesaro S, Maschmeyer G, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71:2379–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

