Bemarituzumab plus mFOLFOX6 as first-line treatment in East Asian patients with FGFR2b-overexpressing locally advanced or metastatic gastric/gastroesophageal junction cancer: subgroup of FIGHT final analysis
- PMID: 38861192
- PMCID: PMC11335773
- DOI: 10.1007/s10120-024-01516-3
Bemarituzumab plus mFOLFOX6 as first-line treatment in East Asian patients with FGFR2b-overexpressing locally advanced or metastatic gastric/gastroesophageal junction cancer: subgroup of FIGHT final analysis
Abstract
Background: In the FIGHT study (NCT03694522) bemarituzumab, a humanized monoclonal antibody selective for fibroblast growth factor receptor 2b (FGFR2b), plus mFOLFOX6 showed clinically meaningful efficacy in patients with FGFR2b-positive (2+/3+ membranous staining by immunohistochemistry) locally advanced unresectable/metastatic gastric/gastroesophageal cancer (G/GEJC). A meaningful proportion of patients in FIGHT were enrolled in East Asia, reflecting global epidemiology of G/GEJC.
Methods: This subgroup analysis of the global, phase 2, double-blind FIGHT study included all patients enrolled in East Asian sites. Patients were randomized 1:1 to bemarituzumab-mFOLFOX6 (15 mg/kg and one 7.5 mg/kg dose on cycle 1, day 8) or matching placebo-mFOLFOX6. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate, and safety. Efficacy was evaluated after a minimum follow-up of 24 months.
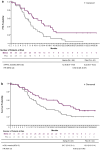
Results: The East Asian subgroup comprised 89 patients (57% of overall study population); 45 were randomized to bemarituzumab-mFOLFOX6 and 44 to placebo-mFOLFOX6. Median PFS (95% confidence interval [CI]) was 12.9 months (8.8-17.9) with bemarituzumab-mFOLFOX6 and 8.2 months (5.6-10.3) with placebo-mFOLFOX6 (HR 0.50, 95% CI 0.29-0.87); median OS (95% CI) was 24.7 months (13.8-33.1) vs 12.9 months (9.3-21.4), respectively (HR 0.56, 95% CI 0.32-0.96). Treatment benefit was more pronounced in patients with FGFR2b-positive G/GEJC in ≥ 10% of tumor cells. No new safety signals were reported.
Conclusion: In East Asian patients with FGFR2b-positive advanced/metastatic G/GEJC enrolled in the global FIGHT study, bemarituzumab-mFOLFOX6 showed clinically meaningful outcomes over placebo-mFOLFOX6.
Keywords: Bemarituzumab; FGFR2b; Gastric cancer; Targeted therapy; mFOLFOX6.
© 2024. The Author(s).
Conflict of interest statement
Yoon-Koo Kang: Consulting fees (self): Amgen, Novartis, Roche, Daehwa, Zymeworks, Blueprint, Surface Oncology, ALX Oncology, Macrogenics, BMS, Merck, Liscure. Shukui Qin: None to disclose. Keun-Wook Lee: All support for the present manuscript: Five Prime Therapeutics; Grants or contracts from any entity: All to institution for conducting clinical trials—AstraZeneca, Ono pharmaceutical, Merck Sharp and Dohme, Merck KGaA, Roche, Pfizer, Beigene, Leap therapeutics, ALX Oncology, Zymeworks, Astellas, Macrogenics, Amgen, Seagen, Bolt therapeutics, Trishula therapeutics, Oncologie, Pharmacyclics, MedPacto, Green Cross Corp, ABLBIO, Y-BIOLOGICS, Daiichi Sankyo, Taiho Pharmaceutical, InventisBio, Elevar Therapeutics, Metafines, Idience, Genome & Company, Exelixis; Honoraria for lectures: Ono pharmaceutical, Boryung, Daiichi Sankyo, Astellas, Sanofi-Aventis; Participation on a Data Safety Monitoring Board or Advisory Board: ALX Oncology, Metafines. Sang Cheul Oh: None to disclose. In-Ho Kim: None to disclose. Jong Gwang Kim: None to disclose. Yong Li: None to disclose. Zhuchen Yan: None to disclose. Jin Li: Research Grants: Roche; Speakers Bureau: Eli Lilly, AstraZeneca. Li-Yuan Bai: None to disclose. Catherine Chan: Employee and stockholder, Amgen Inc. Akeem Yusuf: Employee and stockholder, Amgen Inc. Anita Zalten-Kümeli: Employee and stockholder, Amgen Inc. Kate Taylor: Employee and stockholder, Amgen Inc. Kensei Yamaguchi: Research Grants: Taiho Pharm; Speakers Bureau: Daiichi Sankyo Co., Ltd.,Chugai Pharmaceutical Co., Ltd.,Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Biopharm Co., Ltd.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

