Recent advances in the application of [2 + 2] cycloaddition in the chemical synthesis of cyclobutane-containing natural products
- PMID: 38861197
- PMCID: PMC11166626
- DOI: 10.1007/s13659-024-00457-9
Recent advances in the application of [2 + 2] cycloaddition in the chemical synthesis of cyclobutane-containing natural products
Abstract
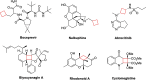
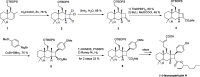
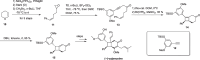
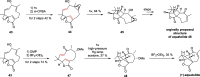
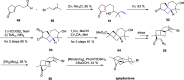
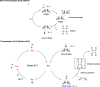
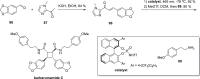
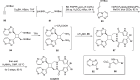
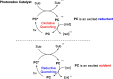
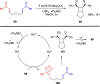
Cyclobutanes are distributed widely in a large class of natural products featuring diverse pharmaceutical activities and intricate structural frameworks. The [2 + 2] cycloaddition is unequivocally the primary and most commonly used method for synthesizing cyclobutanes. In this review, we have summarized the application of the [2 + 2] cycloaddition with different reaction mechanisms in the chemical synthesis of selected cyclobutane-containing natural products over the past decade.
Keywords: Cyclobutane; Natural products; Total synthesis; [2 + 2] Cycloaddition.
© 2024. The Author(s).
Conflict of interest statement
All the authors declare that there is no competitive interest related to this work.
Figures
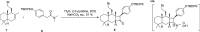

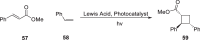
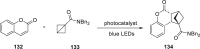
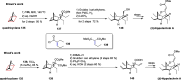
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

