Development of a Novel Patient-Reported Outcome Measure to Assess Symptoms and Impacts of Androgen Deprivation Therapy for Advanced Prostate Cancer
- PMID: 38861216
- PMCID: PMC11263404
- DOI: 10.1007/s12325-024-02888-9
Development of a Novel Patient-Reported Outcome Measure to Assess Symptoms and Impacts of Androgen Deprivation Therapy for Advanced Prostate Cancer
Abstract
Introduction: This qualitative research study was conducted to develop a novel, comprehensive, patient-reported outcome measure (PRO), the "Symptoms and Impacts of Androgen Deprivation Therapy (ADT) for Prostate Cancer" (SIADT-PC), assessing hormonal therapy-related symptoms and their impacts on men with advanced prostate cancer.
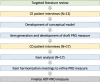
Methods: Concept elicitation (CE) interviews were conducted among adult men with prostate cancer to evaluate their experiences with ADT. Based on key symptom and impact concepts mentioned, an initial PRO measure was developed. The draft measure was further assessed in cognitive debriefing (CD) interviews with men with prostate cancer, in which participants reviewed items, response options, and recall periods. Initial item-based psychometric analyses were conducted using interview data. The draft questionnaire was revised on the basis of participant feedback, quantitative psychometric results, and consultation with clinical experts.
Results: A total of 21 participants were interviewed (CE concept elicitation, n = 12; CD cognitive debriefing, n = 17; n = 8 completed both). Mean participant age (SD) was 59.7 (8.7) years and 76.2% were white. The de novo SIADT-PC measure consists of 27 items: 11 symptoms (e.g., fatigue, hot flashes, and erectile dysfunction), 2 long-term symptoms (e.g., weight gain), 10 impacts (e.g., impacts on physical activities and relationships), and 4 related to mode of administration (i.e., injection-site reactions). Items were assessed with a 5-point verbal rating scale, with answer choices that capture frequency or severity.
Conclusions: Once fully validated, this de novo measure may be used in clinical studies and clinical practice to assess hormone therapy-related symptoms and impacts, enabling physicians to identify timely and appropriate interventions.
Keywords: Advanced prostate cancer; Androgen deprivation therapy; Gonadotropin-releasing hormone; Health-related quality of life; Luteinizing hormone-releasing hormone; Patient-reported outcome measure.
© 2024. The Author(s).
Conflict of interest statement
Bryan Selby (Senior VP, Product Development, was an employee of Myovant Sciences Inc, Employee, Brisbane, CA); Michael Chladek and Ruby Gogana (were employees of Clinical Outcomes Solutions, COS, Chicago, IL, US; COS was supported by Sumitomo Pharma Switzerland GmbH). Ashley E. Ross is a consultant for Veracyte Inc., Astellas Pharma Inc., Bayer, Blue Earth Inc., Janssen, Lantheus, and Tempus. Kelsie Brewer was an employee of Clinical Outcomes Solutions at the time this study was done. Stacie Hudgens is an employee of Clinical Outcomes Solutions. Bruce Brown was an employee of Myovant Sciences Inc. at the time this study was done. Mark Fallick was an employee of Myovant Sciences Inc. at the time this study was done. Simon dePaauw-Holt is an employee of Sumitomo Pharma Switzerland GmbH. Bhakti Arondekar is an employee of and owns stock in Pfizer, Inc. Jennifer Clegg is an employee of Clinical Outcomes Solutions. Elke Hunsche is an employee of Sumitomo Pharma Switzerland GmbH.
Figures
References
-
- National Cancer Institute. Cancer stat facts: prostate cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed March 12, 2023.
-
- American Cancer Society. Prostate cancer early detection, diagnosis, and staging. Updated August 1, 2019. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-stagin.... Accessed December 30, 2022.
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Prostate Cancer. Version 1.2023; 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical