De-escalation of Disease-Modifying Therapy for People with Multiple Sclerosis Due to Safety Considerations: Characterizing 1-Year Outcomes in 25 People Who Switched from Ocrelizumab to Diroximel Fumarate
- PMID: 38861218
- PMCID: PMC11263251
- DOI: 10.1007/s12325-024-02902-0
De-escalation of Disease-Modifying Therapy for People with Multiple Sclerosis Due to Safety Considerations: Characterizing 1-Year Outcomes in 25 People Who Switched from Ocrelizumab to Diroximel Fumarate
Abstract
Introduction: Switching disease-modifying therapy (DMT) may be considered for relapsing-remitting multiple sclerosis (RRMS) if a patient's current therapy is no longer optimal. This was particularly important during the recent COVID-19 pandemic because of considerations around immune deficiency and impaired vaccine response associated with B cell-depleting DMTs. This real-world, single-center study aimed to evaluate change or decline in functional ability and overall disease stability in people with RRMS who were switched from B cell-depleting ocrelizumab (OCRE) to diroximel fumarate (DRF) because of safety concern related to the COVID-19 pandemic.
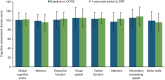
Methods: Adults with RRMS were included if they had been clinically stable for ≥ 1 year on OCRE. Data collected at baseline and 1 year post switch included relapse rate, magnetic resonance imaging (MRI), blood work for assessment of peripheral immune parameters, the Cognitive Assessment Battery (CAB), optical coherence tomography (OCT), and patient-reported outcomes (PROs).
Results: Participants (N = 25) had a mean (SD) age of 52 (9) years, and a mean (SD) duration of 26 (8) months' treatment with OCRE before the switch to DRF. Median washout duration since the last OCRE infusion was 7 months (range 4-18 months). No participants relapsed on DRF during follow-up, and all remained persistent on DRF after 1 year. There were no significant changes in peripheral immune parameters, other than an increase in the percentage of CD19+ cells 1 year after switching (p < 0.05). Similarly, there were no significant changes in CAB, OCT, and PROs.
Conclusion: These preliminary findings suggest that transition to DRF from OCRE may be an effective treatment option for people with RRMS who are clinically stable but may need to switch for reasons unrelated to effectiveness. Longer follow-up times on larger samples are needed to confirm these observations.
Keywords: Cognitive Assessment Battery; Diroximel fumarate; Ocrelizumab; Ocular tomography; Patient-reported outcomes; Relapsing–remitting multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
Mark Gudesblatt: speaker and consulting for Acorda, Amgen, Biogen, EMD Serono, Medtronic, Novartis, Sanofi Genzyme, Saol Therapeutics, and Teva Pharmaceuticals. Daniel Golan: Speaker and consulting for Bristol Myers Squib, Medison, Merck Serono, Novartis, and Roche. Barbara Bumstead: speaker and consulting for Biogen and BMS. Marijean Buhse: speaker and consulting for Biogen and Genzyme. Myassar Zarif: speaker and consulting for Biogen, BMS, EMD Serono, Horizon, Genentech, Novartis, and Sanofi. Sarah A. Morrow: served on advisory boards for Biogen Idec, EMD Serono, Novartis, Roche, and Sanofi Genzyme; received investigator-initiated grant funds from Biogen Idec, Roche, and Sanofi Genzyme; acted as site PI for multi-center trials funded by BMS, EMD Serono, Novartis, Roche, and Sanofi Genzyme; and received funding from CIHR, MSSC, and NMSS. Dr. Morrow’s new affiliation is Department of Clinical Neurosciences, University of Calgary, Hotchkiss Brain Institute, Calgary, AB, Canada. The affiliation listed in the title was Dr. Morrow’s affiliation at the time of the study. Jacqueline A. Nicholas: has received consultancy fees from Bristol Myers Squibb, EMD Serono, Genentech, MyMSTeam, Novartis, Octave Bio and TG Therapeutics; has received research support from Novartis, Genentech, Alexion, Octave Bio, Sanofi Genzyme, University of Buffalo and PCORI; serves on speakers’ bureau for EMD Serono and TG Therapeutics. Laura M. Hancock: research support from BMS, Clinical & Translational Science Institute and Advancing a Healthier Wisconsin Research and Education Program, and NIH; and speaker honoraria from Can Do MS, the. MS Association of America, and the National MS Society of America. Jeffrey Wilken: nothing to disclose. Nicole Scott, Anne Gocke, James B. Lewin, and Jason P. Mendoza: employees of and hold stock/stock options in Biogen. Olivia Kaczmarek: nothing to disclose. Joanna Weller: nothing to disclose.
Figures

Similar articles
-
Health-Related Quality of Life with Diroximel Fumarate in Patients with Relapsing Forms of Multiple Sclerosis: Findings from Qualitative Research Using Patient Interviews.Adv Ther. 2022 Jul;39(7):3199-3213. doi: 10.1007/s12325-022-02164-8. Epub 2022 May 13. Adv Ther. 2022. PMID: 35556227 Free PMC article.
-
Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: A multicenter retrospective observational study (STRATEGY).Mult Scler Relat Disord. 2018 May;22:27-34. doi: 10.1016/j.msard.2018.02.028. Epub 2018 Feb 26. Mult Scler Relat Disord. 2018. PMID: 29524759 Clinical Trial.
-
Safety, tolerability, and efficacy of diroximel fumarate in a cohort of Black patients with multiple sclerosis from the phase 3 EVOLVE-MS-1 study.Mult Scler Relat Disord. 2024 Nov;91:105912. doi: 10.1016/j.msard.2024.105912. Epub 2024 Sep 30. Mult Scler Relat Disord. 2024. PMID: 39393172 Clinical Trial.
-
Real-world effectiveness, safety and immunogenicity of ocrelizumab in turkish multiple sclerosis patients: a single-center experience for 4-year follow-up.Acta Neurol Belg. 2024 Aug;124(4):1385-1391. doi: 10.1007/s13760-024-02572-3. Epub 2024 May 20. Acta Neurol Belg. 2024. PMID: 38769274 Review.
-
Efficacy, safety and patient reported outcomes in patients with active relapsing multiple sclerosis treated with ocrelizumab: Final results from the PRO-MSACTIVE study.Mult Scler Relat Disord. 2022 Dec;68:104109. doi: 10.1016/j.msard.2022.104109. Epub 2022 Aug 13. Mult Scler Relat Disord. 2022. PMID: 36007299 Review.
Cited by
-
Clinical characteristics and treatment outcomes in multiple sclerosis patients treated with anti-CD20s who switched to fumarates: a retrospective analysis of a US healthcare claims database.J Comp Eff Res. 2025 Mar;14(3):e240071. doi: 10.57264/cer-2024-0071. Epub 2025 Feb 12. J Comp Eff Res. 2025. PMID: 39936542 Free PMC article.
-
Glial Cells as Key Regulators in Neuroinflammatory Mechanisms Associated with Multiple Sclerosis.Int J Mol Sci. 2024 Sep 4;25(17):9588. doi: 10.3390/ijms25179588. Int J Mol Sci. 2024. PMID: 39273535 Free PMC article. Review.
-
De-Escalation of Disease-Modifying Therapy in Multiple Sclerosis-A Danish Nationwide Cohort Study.Eur J Neurol. 2025 Feb;32(2):e70042. doi: 10.1111/ene.70042. Eur J Neurol. 2025. PMID: 39895213 Free PMC article.
-
Safety and effectiveness of diroximel fumarate in relapsing forms of multiple sclerosis: a systematic review and meta-analysis.Neurol Sci. 2025 Aug;46(8):3477-3490. doi: 10.1007/s10072-025-08140-8. Epub 2025 Apr 14. Neurol Sci. 2025. PMID: 40229470 Free PMC article.
References
-
- National Multiple Sclerosis Society. Disease-modifying therapies for MS 2022. https://www.nationalmssociety.org/managingms/treating-ms/disease-modifyi.... Accessed 9 Dec 2022
-
- Burtchell J, Clemmons D, Clemmons J, et al. A targeted literature search and phenomenological review of perspectives of people with multiple sclerosis and healthcare professionals of the immunology of disease-modifying therapies. Neurol Ther. 2022;11(3):955–79. 10.1007/s40120-022-00349-5 - DOI - PMC - PubMed
-
- Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord. 2018;22:27–34. 10.1016/j.msard.2018.02.028 - DOI - PubMed
-
- Kresa-Reahl K, Repovic P, Robertson D, Okwuokenye M, Meltzer L, Mendoza JP. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–87. 10.1016/j.clinthera.2018.10.011 - DOI - PubMed
-
- Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88. 10.1212/WNL.0000000000005347 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

