Protein Disulfide Isomerase Endoplasmic Reticulum Protein 57 (ERp57) is Protective Against ALS-Associated Mutant TDP-43 in Neuronal Cells
- PMID: 38861223
- PMCID: PMC11166824
- DOI: 10.1007/s12017-024-08787-0
Protein Disulfide Isomerase Endoplasmic Reticulum Protein 57 (ERp57) is Protective Against ALS-Associated Mutant TDP-43 in Neuronal Cells
Abstract
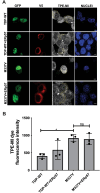
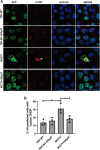
Amyotrophic Lateral Sclerosis (ALS) is a severe neurodegenerative disease affecting motor neurons. Pathological forms of Tar-DNA binding protein-43 (TDP-43), involving its mislocalisation to the cytoplasm and the formation of misfolded inclusions, are present in almost all ALS cases (97%), and ~ 50% cases of the related condition, frontotemporal dementia (FTD), highlighting its importance in neurodegeneration. Previous studies have shown that endoplasmic reticulum protein 57 (ERp57), a member of the protein disulphide isomerase (PDI) family of redox chaperones, is protective against ALS-linked mutant superoxide dismutase (SOD1) in neuronal cells and transgenic SOD1G93A mouse models. However, it remains unclear whether ERp57 is protective against pathological TDP-43 in ALS. Here, we demonstrate that ERp57 is protective against key features of TDP-43 pathology in neuronal cells. ERp57 inhibited the mislocalisation of TDP-43M337V from the nucleus to the cytoplasm. In addition, ERp57 inhibited the number of inclusions formed by ALS-associated variant TDP-43M337V and reduced the size of these inclusions. ERp57 was also protective against ER stress and induction of apoptosis. Furthermore, ERp57 modulated the steady-state expression levels of TDP-43. This study therefore demonstrates a novel mechanism of action of ERp57 in ALS. It also implies that ERp57 may have potential as a novel therapeutic target to prevent the TDP-43 pathology associated with neurodegeneration.
Keywords: ALS—Amyotrophic lateral sclerosis; ER stress; ERp57—Endoplasmic reticulum protein 57; PDI—Protein disulphide isomerase; TDP-43 pathology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Acewicz, A., Stępień, T., Felczak, P., Tarka, S., & Wierzba-Bobrowicz, T. (2022). Incidence and morphology of secondary TDP-43 proteinopathies: Part 1. Folia Neuropathologica,60(3), 267–276. 10.5114/fn.2022.120314 - PubMed
-
- Barmada, S. J., Serio, A., Arjun, A., Bilican, B., Daub, A., Ando, D. M., Tsvetkov, A., Pleiss, M., Li, X., Peisach, D., Shaw, C., Chandran, S., & Finkbeiner, S. (2014). Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nature Chemical Biology,10(8), 677–685. 10.1038/nchembio.1563 - PMC - PubMed
-
- Barmada, S. J., Skibinski, G., Korb, E., Rao, E. J., Wu, J. Y., & Finkbeiner, S. (2010). Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. Journal of Neuroscience,30(2), 639–649. 10.1523/jneurosci.4988-09.2010 - PMC - PubMed
-
- Bilches Medinas, D., Malik, S., Yildiz-Bölükbasi, E., Borgonovo, J., Saaranen, M. J., Urra, H., Pulgar, E., Afzal, M., Contreras, D., Wright, M. T., Bodaleo, F., Quiroz, G., Rozas, P., Mumtaz, S., Díaz, R., Rozas, C., Cabral-Miranda, F., Piña, R., Valenzuela, V., … Tolun, A. (2022). Mutation in protein disulfide isomerase A3 causes neurodevelopmental defects by disturbing endoplasmic reticulum proteostasis. EMBO Journal,41(2), e105531. 10.15252/embj.2020105531 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

