Dry powder inhaler design and particle technology in enhancing Pulmonary drug deposition: challenges and future strategies
- PMID: 38861247
- PMCID: PMC11555000
- DOI: 10.1007/s40199-024-00520-3
Dry powder inhaler design and particle technology in enhancing Pulmonary drug deposition: challenges and future strategies
Abstract
Objectives: The efficient delivery of drugs from dry powder inhaler (DPI) formulations is associated with the complex interaction between the device design, drug formulations, and patient's inspiratory forces. Several challenges such as limited emitted dose of drugs from the formulation, low and variable deposition of drugs into the deep lungs, are to be resolved for obtaining the efficiency in drug delivery from DPI formulations. The objective of this study is to review the current challenges of inhaled drug delivery technology and find a way to enhance the efficiency of drug delivery from DPIs.
Methods/evidence acquisition: Using appropriate keywords and phrases as search terms, evidence was collected from the published articles following SciFinder, Web of Science, PubMed and Google Scholar databases.
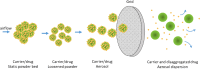
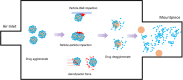
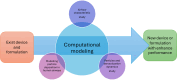
Results: Successful lung drug delivery from DPIs is very challenging due to the complex anatomy of the lungs and requires an integrated strategy for particle technology, formulation design, device design, and patient inhalation force. New DPIs are still being developed with limited performance and future device design employs computer simulation and engineering technology to overcome the ongoing challenges. Many issues of drug formulation challenges and particle technology are concerning factors associated with drug dispersion from the DPIs into deep lungs.
Conclusion: This review article addressed the appropriate design of DPI devices and drug formulations aligned with the patient's inhalation maneuver for efficient delivery of drugs from DPI formulations.
Keywords: Computational modelling; DPI device design; Drug formulation; Lung deposition; Particle technology.
© 2024. The Author(s), under exclusive licence to Tehran University of Medical Sciences.
Conflict of interest statement
Figures

Similar articles
-
The clinical relevance of dry powder inhaler performance for drug delivery.Respir Med. 2014 Aug;108(8):1195-203. doi: 10.1016/j.rmed.2014.05.009. Epub 2014 May 24. Respir Med. 2014. PMID: 24929253
-
Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part II: Dry powder inhaler application.Comput Biol Med. 2017 May 1;84:247-253. doi: 10.1016/j.compbiomed.2016.10.025. Epub 2016 Nov 3. Comput Biol Med. 2017. PMID: 27836120
-
Dry powder inhalation: past, present and future.Expert Opin Drug Deliv. 2017 Apr;14(4):499-512. doi: 10.1080/17425247.2016.1224846. Epub 2016 Aug 30. Expert Opin Drug Deliv. 2017. PMID: 27534768 Review.
-
Dry powder inhalers in COPD, lung inflammation and pulmonary infections.Expert Opin Drug Deliv. 2015 Jun;12(6):947-62. doi: 10.1517/17425247.2015.977783. Epub 2014 Nov 12. Expert Opin Drug Deliv. 2015. PMID: 25388926 Review.
-
Building respirable powder architectures: utilizing polysaccharides for precise control of particle morphology for enhanced pulmonary drug delivery.Expert Opin Drug Deliv. 2024 Jun;21(6):945-963. doi: 10.1080/17425247.2024.2376702. Epub 2024 Jul 8. Expert Opin Drug Deliv. 2024. PMID: 38961522 Review.
Cited by
-
Zanamivir exposure in healthy rats and rats with acute lung injury.Ann Med. 2025 Dec;57(1):2534523. doi: 10.1080/07853890.2025.2534523. Epub 2025 Jul 20. Ann Med. 2025. PMID: 40684449 Free PMC article.
-
Optimization, In Vitro, and In Silico Characterization of Theophylline Inhalable Powder Using Raffinose-Amino Acid Combination as Fine Co-Spray-Dried Carriers.Pharmaceutics. 2025 Apr 3;17(4):466. doi: 10.3390/pharmaceutics17040466. Pharmaceutics. 2025. PMID: 40284461 Free PMC article.
-
Inhalable nanoparticle-based delivery systems for the treatment of pulmonary infections: Status quo and barrier-overcoming strategies.Drug Deliv. 2025 Dec;32(1):2544683. doi: 10.1080/10717544.2025.2544683. Epub 2025 Aug 11. Drug Deliv. 2025. PMID: 40790871 Free PMC article. Review.
-
Development and Characterization of Spray-Dried Combined Levofloxacin-Ambroxol Dry Powder Inhaler Formulation.Pharmaceutics. 2024 Nov 22;16(12):1506. doi: 10.3390/pharmaceutics16121506. Pharmaceutics. 2024. PMID: 39771486 Free PMC article.
References
-
- Tse JY, Koike A, Kadota K, Uchiyama H, Fujimori K, Tozuka Y. Porous particles and novel carrier particles with enhanced penetration for efficient pulmonary delivery of antitubercular drugs. Eur J Pharm Biopharm. 2021;167:116–26. - PubMed
-
- Ali AM, Abo Dena AS, Yacoub MH, El-Sherbiny IM. Exploring the influence of particle shape and air velocity on the flowability in the respiratory tract: a computational fluid dynamics approach. Drug Dev Ind Pharm. 2019;45(7):1149–56. - PubMed
-
- Gaikwad SS, Pathare SR, More MA, Waykhinde NA, Laddha UD, Salunkhe KS, Kshirsagar SJ, Patil SS, Ramteke KH. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J Control Release. 2023;355:292–311. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

