Peptide-Hitchhiking for the Development of Nanosystems in Glioblastoma
- PMID: 38861272
- PMCID: PMC11223498
- DOI: 10.1021/acsnano.4c01790
Peptide-Hitchhiking for the Development of Nanosystems in Glioblastoma
Abstract
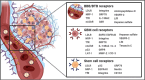
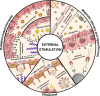
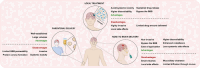
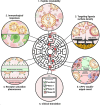
Glioblastoma (GBM) remains the epitome of aggressiveness and lethality in the spectrum of brain tumors, primarily due to the blood-brain barrier (BBB) that hinders effective treatment delivery, tumor heterogeneity, and the presence of treatment-resistant stem cells that contribute to tumor recurrence. Nanoparticles (NPs) have been used to overcome these obstacles by attaching targeting ligands to enhance therapeutic efficacy. Among these ligands, peptides stand out due to their ease of synthesis and high selectivity. This article aims to review single and multiligand strategies critically. In addition, it highlights other strategies that integrate the effects of external stimuli, biomimetic approaches, and chemical approaches as nanocatalytic medicine, revealing their significant potential in treating GBM with peptide-functionalized NPs. Alternative routes of parenteral administration, specifically nose-to-brain delivery and local treatment within the resected tumor cavity, are also discussed. Finally, an overview of the significant obstacles and potential strategies to overcome them are discussed to provide a perspective on this promising field of GBM therapy.
Keywords: biomimetic approaches; blood−brain barrier (BBB); external stimuli; extracellular vesicles; glioblastoma; local treatment; nanocatalytic medicine; nanoparticles; nose-to-brain delivery; peptide functionalization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Nanosystems at Nexus: Navigating Nose-to-Brain Delivery for Glioblastoma Treatment.Mol Pharm. 2025 Feb 3;22(2):599-619. doi: 10.1021/acs.molpharmaceut.4c00703. Epub 2025 Jan 2. Mol Pharm. 2025. PMID: 39746097 Review.
-
Classical Monocytes Shuttling for Precise Delivery of Nanotherapeutics to Glioblastoma.Adv Healthc Mater. 2024 Nov;13(29):e2400925. doi: 10.1002/adhm.202400925. Epub 2024 Aug 30. Adv Healthc Mater. 2024. PMID: 39212635
-
Recent advances in targeted drug delivery for the treatment of glioblastoma.Nanoscale. 2024 May 9;16(18):8689-8707. doi: 10.1039/d4nr01056f. Nanoscale. 2024. PMID: 38606460 Review.
-
Advanced nanomicelles for targeted glioblastoma multiforme therapy.Biomater Adv. 2025 May;170:214221. doi: 10.1016/j.bioadv.2025.214221. Epub 2025 Feb 4. Biomater Adv. 2025. PMID: 39922136 Review.
-
Charge-switchable cell-penetrating peptides for rerouting nanoparticles to glioblastoma treatment.Colloids Surf B Biointerfaces. 2024 Sep;241:113983. doi: 10.1016/j.colsurfb.2024.113983. Epub 2024 May 28. Colloids Surf B Biointerfaces. 2024. PMID: 38850741
Cited by
-
Navigating the brain: Harnessing endogenous cellular hitchhiking for targeting neoplastic and neuroinflammatory diseases.Asian J Pharm Sci. 2025 Apr;20(2):101040. doi: 10.1016/j.ajps.2025.101040. Epub 2025 Feb 26. Asian J Pharm Sci. 2025. PMID: 40453753 Free PMC article. Review.
-
Exosomes in the Chemoresistance of Glioma: Key Point in Chemoresistance.J Cell Mol Med. 2025 Feb;29(4):e70401. doi: 10.1111/jcmm.70401. J Cell Mol Med. 2025. PMID: 39950738 Free PMC article. Review.
-
Peptide-Based Nanoparticle for Tumor Therapy.Biomedicines. 2025 Jun 9;13(6):1415. doi: 10.3390/biomedicines13061415. Biomedicines. 2025. PMID: 40564134 Free PMC article. Review.
-
Better together: biomimetic nanomedicines for high performance tumor therapy.Beilstein J Nanotechnol. 2025 Aug 5;16:1246-1276. doi: 10.3762/bjnano.16.92. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 40791939 Free PMC article. Review.
-
Recent Treatment Strategies and Molecular Pathways in Resistance Mechanisms of Antiangiogenic Therapies in Glioblastoma.Cancers (Basel). 2024 Aug 27;16(17):2975. doi: 10.3390/cancers16172975. Cancers (Basel). 2024. PMID: 39272834 Free PMC article. Review.
References
-
- Janjua T. I.; Rewatkar P.; Ahmed-Cox A.; Saeed I.; Mansfeld F. M.; Kulshreshtha R.; Kumeria T.; Ziegler D. S.; Kavallaris M.; Mazzieri R.; Popat A. Frontiers in the Treatment of Glioblastoma: Past, Present and Emerging. Adv. Drug Delivery Rev. 2021, 171, 108–138. 10.1016/j.addr.2021.01.012. - DOI - PubMed
-
- Stupp R.; Mason W. P.; Van Den Bent M. J.; Weller M.; Fisher B.; Taphoorn M. J. B.; Belanger K.; Brandes A. A.; Marosi C.; Bogdahn U.; Curschmann J.; Janzer R. C.; Ludwin S. K.; Gorlia T.; Allgeier A.; Lacombe D.; Cairncross J. G.; Eisenhauer E.; Mirimanoff R. O. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine 2005, 352, 987–996. 10.1056/NEJMoa043330. - DOI - PubMed
-
- Stupp R.; Hegi M. E.; Mason W. P.; van den Bent M. J.; Taphoorn M. J.; Janzer R. C.; Ludwin S. K.; Allgeier A.; Fisher B.; Belanger K.; Hau P.; Brandes A. A.; Gijtenbeek J.; Marosi C.; Vecht C. J.; Mokhtari K.; Wesseling P.; Villa S.; Eisenhauer E.; Gorlia T.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol 2009, 10, 459–466. 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

